Abstract
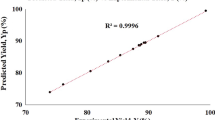
Synthesis of FAME from Brassica carinata oil to produce biodiesel was accomplished using potassium hydroxide as the catalyst. A factorial design of experiments and a central composite design were used. The variables chosen were: type of Brassica carinata oil, initial catalyst concentration, and temperature; and the responses were FAME purity and yield. The type of B. carinata oil included high-erucic B. carinata (HEBC) and lowerucic B. carinata (LEBC) varieties. The results show that the type of B. carinata oil does not affect the purity and yield of FAME. However, HEBC oil is more suitable for biodiesel production because its iodine value is lower and within the European Union specifications. The initial catalyst concentration is the most important factor, having a positive influence on FAME purity but a negative effect on FAME yield. The temperature has a significant positive effect on FAME purity and a significant negative influence on FAME yield. Second-order models were obtained to predict FAME purity and yield as a function of catalyst concentration and temperature for HEBC oil methanolysis. The best conditions for this process are 25°C, and 1.2–1.5 wt% for the catalyst concentration.
Similar content being viewed by others
References
Vicente, G., M. Martínez, and J. Aracil, Integrated Biodiesel Production: A Comparison of Different Homogeneous Catalysts Systems, Bioresour. Technol. 92:297–305 (2004).
Freedman, B., E.H. Pryde, and T.L. Mounts, Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils, J. Am. Oil Chem. Soc. 61:1638–1643 (1984).
Reid, E.E., Studies in Esterification. IV. The Interdependence of Limits as Exemplified in the Transformation of Esters, Am. Chem. J. 45:479–516 (1911).
Mittelbach, M., M. Woergetter, J. Pernkopf, and H. Junek, Diesel Fuel Derived from Vegetable Oils: Preparation and Use of Rape Oil Methyl Ester, Energy Agric. 2:369–384 (1983).
Vicente, G., A. Coteron, M. Martínez, and J. Aracil, Application of the Factorial Design of Experiments and Response Surface Methodology to Optimize Biodiesel Production, Ind. Crop. Prod. 8:29–35 (1998).
Getinet, A., G. Raskow, J.P. Raney, and R.K. Downey, Development of Zero Erucic Acid Ethiopian Mustard Through an Interspecific Cross with Zero Erucic Oriental Mustard, Can. J. Plant Sci. 74:793–795 (1994).
Velasco, L., J.M. Fernández-Martínez, and A. De Haro. Inheritance of Increased Oleic Acid Concentration in High-Erucic Acid Ethiopian Mustard, Crop Sci. 43:106–109 (2003).
Cardone, M., M.V. Prati, V. Rocco, M. Seggiani, A. Senatore, and S. Vitolo, Brassica carinata as an Alternative Oil Crop for the Production of Biodiesel in Italy: Engine Performance and Regulated and Unregulated Exhaust Emissions, Environ. Sci. Technol. 36:4656–4662 (2002).
Cardone, M., M. Mazzoncini, S. Menini, V. Rocco, A. Senatore, M. Seggiani, and S. Vitolo, Brassica carinata Oil as an Alternative Oil Crop for the Production of Biodiesel in Italy: Agronomic Evaluation, Fuel Production of Transesterification and Characterization, Biomass Bioenerg. 25:623–636 (2003).
Coterón, A., N. Sánchez, M. Martínez, and J. Aracil, Optimization of the Synthesis of an Analogue of Jojoba Oil Using a Fully Central Composite Design, Can. J. Chem. Eng. 71:485–488 (1993).
García, D., T. García, A. Coterón, M. Martínez, and J. Aracil, Optimization of the Synthesis of Sperm Whale Oil Analogue, Ind. Crop. Prod. 4:105–111 (1995).
Sánchez, N., M. Martínez, and J. Aracil, Selective Esterification of Glycerine to 1-Glycerol Monooleate. 2. Optimization Studies, Ind. Eng. Chem. Res. 36:1529–1534 (1997).
Method Ca 5a–40: Free Fatty Acids; Method Cd 3–25: Saponification Value; Method Cd 1–25: Iodine Value of Fats and Oils; Wijs Method. Method Cd 8–53: Peroxide Value Acetic Acid-Chloroform Method in Official Methods and Recommended Practices of the AOCS, 5th edn., edited by D. Firestone, AOCS Press, Champaign, 1997.
Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density Hydrometer Method (ISO 3675: 1998), European Standard EN ISO 3675, CEN European Committee for Standardization, Brussels, 1998.
Petroleum Products, Transparent and Opaque Liquids. Determination of Kinetic Viscosity and Calculation of Dynamic Viscosity (ISO 3104: 1994), European Stadard EN ISO 3104, CEN European Committee for Standardization, Brussels, 1996.
Petroleum Products, Determination of Water. Coulometric Karl Fischer Titration Method (ISO 12937: 2000), European Standard EN ISO 12937, CEN European Committee for Standardization, Brussels, 2000.
Bailer, J.Y., and K. de Hueber, Enzymatical Determination of Free Glycerol and Bonded Glycerol, in Handbook of Analytical Methods for Fatty Acid Methyl Esters Used as Diesel Fuel, Forschungsinstitut für Chemie und Technologie von Erdölprodukten (IFICHTE) Institute, Vienna, 1994, pp. 19–20.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Vicente, G., Martínez, M. & Aracil, J. Optimization of Brassica carinata oil methanolysis for biodiesel production. J Amer Oil Chem Soc 82, 899–904 (2005). https://doi.org/10.1007/s11746-005-1162-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-005-1162-6