Abstract
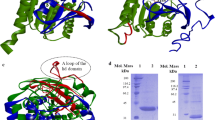
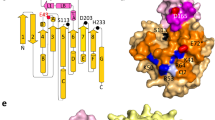
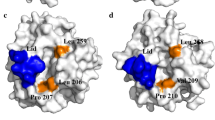
The acyl binding site of Rhizopus delemar prolipase and mature lipase was altered through site-directed mutagenesis to improve lipase specificity for short-or medium-chain length fatty acids. Computer-generated structural models of R. delemar lipase were used in mutant protein design and in the interpretation of the catalytic properties of the resulting recombinant enzymes. Molecular dynamics simulations of the double mutant, val209trp+phe112trp, predicted that the introduction of trp112 and trp209 in the acyl binding groove would sterically hinder the docking of fatty acids longer than butyric acid. Assayed against a mixture of triacylglycerol substrates, the val209trp+phe112trp mature lipase mutant showed an 80-fold increase in the hydrolysis of tributyrin relative to the hydrolysis of tricaprylin while no triolein hydrolysis was detected. By comparison, the val94Trp mutant, predicted to pose steric or geometric constraints for docking fatty acids longer than caprylic acid in the acyl binding groove, resulted in a modest 1.4-fold increase in tricaprylin hydrolysis relative to the hydrolysis of tributyrin. Molecular models of the double mutant phe95asp+phe214arg indicated the creation of a salt bridge between asp95 and arg214 across the distal end of the acyl binding groove. When challenged with a mixture of triacylglycerols, the phe95asp+phe214arg substitutions resulted in an enzyme with 3-fold enhanced relative activity for tricaprylin compared to triolein, suggesting that structural determinants for medium-chain length specificity may reside in the distal end of the acyl binding groove. Attempts to introduce a salt bridge within 8 Å of the active site by the double mutation leu146lys+ser115asp destroyed catalytic activity entirely. Similarly, the substitution of polar Gln at the rim of the acyl binding groove for phe 112 largely eliminated catalytic activity of the lipase.
Similar content being viewed by others
Abbreviations
- cDNA:
-
complementary DNA
- IPTG:
-
isopropyl-b-d-thiogalactoside
- Phe:
-
phenylalanine
- Rd :
-
Rhizopus delemar
- TB:
-
tributyrin
- TC:
-
tricaprylin
- TL:
-
trilaurin
- TO:
-
triolein
- Tris:
-
ris(hydroxymethyl)aminomethane
- val:
-
valine
References
Sarda, L., and Desnuelle, P. (1958) Action of Pancreatic Lipase on Esters in Emulsion, Biochim. Biophys. Acta 30, 513–521.
Derewenda, Z.S., and Sharp, A.M. (1993) News from the Interface: The Molecular Structures of Triacylglyceride Lipases, TIBS 18, 20–25.
Derewenda, Z.S. (1995) A Twist in the Tale of Lipolytic Enzymes, Structural Biology 2, 347–349.
Kazlauskas, R.J. (1994) Elucidating Structure-Mechanism Relationships in Lipases: Prospects for Predicting and Engineering Catalytic Properties, TIBTECH 12, 464–472.
Cygler, M., Grochulski, P., and Schrag, J.D. (1995) Structural Determinants Defining Common Stereoselectivity of Lipases Toward Secondary Alcohols, Can. J. Microbiol. 41, 289–296.
Stadler, P., Kovac, A., and Paltauf, F. (1995) Understanding Lipase Action and Selectivity, Croatica Chemica Acta 68, 649–674.
Quinlan, P., and Moore, S. (1993) Modification of Triglycerides by Lipases: Process Technology and its Application to the Production of Nutritionally Improved Fats, INFORM 4, 580–585.
Vulfson, E.N. (1994) Industrial Applications of Lipases, in Lipases, Their Structure, Biochemistry, and Application (Woolley P., and Petersen, S.B., eds.) pp. 271–288, Cambridge University Press, Cambridge.
Haas, M.J., Cichowicz, D.J., and Bailey, D.G. (1992) Purification and Characterization of an Extracellular Lipase from the Fungus Rhizopus delemar, Lipids 27, 571–576.
Haas, M.J., Allen, J., and Berka, T.R. (1991) Cloning, Expression and Characterization of a cDNA Encoding a Lipase from Rhizopus delemar, Gene 109, 107–113.
Joerger, R.D., and Haas, M.J. (1993) Overexpression of a Rhizopus delemar Lipase Gene in Escherichia coli, Lipids 28, 81–87.
Derewenda, U., Swenson, L., Wei, Y., Green, R., Kobos, P.M., Joerger, R., Haas, M.J., and Derewenda, Z.S. (1994) Conformational Lability of Lipases Observed in the Absence of an Oil-Water Interface: Crystallographic Studies of Enzymes from the Fungi Humicola lanuginosa and Rhizopus delemar, J. Lipid Res. 35, 524–534.
Rubin, B. (1994) Crease Pit Chemistry Exposed, Structural Biology, 1, 568–572.
Joerger, R.D., and Haas, M.J. (1994) Alteration of Chain Length Selectivity of a Rhizopus delemar Lipase through Site-Directed Mutagenesis, Lipids 29, 377–384.
Brzozowski, A.M., Derewenda, U., Derewenda, Z.S., Dodson, G.G., Lawson, D.M., Turkenburg, J.P., Bjorkling, F., Huge-Jensen, B., Patkar, S.A., and Thim, L. (1991) A Model for Interfacial Activation in Lipases from the Structure of a Fungal Lipase-Inhibitor Complex, Nature 351, 491–494.
Derewenda, Z.S., Derewenda, U., and Dodson, G.G. (1992) The Crystal and Molecular Structure of the Rhizomucor miehei Triacylglyceride Lipase at 1.9 Å Resolution, J. Mol. Biol. 227, 818–839.
Klein, R.R., and Salvucci, M.E. (1992) Photoaffinity Labeling of Mature and Precursor Forms of the Small Subunit of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase After Expression in Escherichia coli, Plant Physiol. 98, 546–553.
Sturdier, F.W., Rosenberg, A.H., Dunn, J.J., and Dubendorff, J.W. (1991) Use of T7 RNA Polymerase to Direct the Expression of Cloned Genes. Novagen (Technical Bulletin), Madison WI.
Kouker, G., and Jaeger, K.E. (1987) Specific and Sensitive Plate Assay for Bacterial Lipases, App. Environ. Microbiol. 53, 211–213.
Haas, M.J., Esposito, D., and Cichowicz, D.J. (1995) A Software Package to Streamline the Titrimetric Determination of Lipase Activity, J. Am. Oil Chem. Soc. 72, 1405–1406.
Uppenberg, J., Öhrner, N., Norin, M., Hult, K., Kleywegt, G.J., Patkar, S., Waagen, V., Anthonsen, T., and Jones, T.A., (1995) Crystallographic and Molecular-Modeling Studies of Lipase B from Candida antarctica Reveal a Stereospecificity Pocket for Secondary Alcohols, Biochemistry 34, 16838–16851.
Morrissey, J.H. (1981) Silver Stain for Proteins in Polyacrylamide Gels: A Modified Procedure with Enhanced Uniform Sensitivity, Anal. Biochem. 117, 307–310
Holmquist, M., Clausen, I.G., Patkar, S., Svendsen, A., and Hult, K. (1995) Probing a Functional Role of Glu87 and Trp89 in the Lid of Humicola lanuginosa Lipase Through Transesterification Reactions in Organic Solvent, J. Protein Chem. 14, 217–224.
Schmid, R.D., Menge, U., Schomburg, D., and Spener, F. (1995) Towards Novel Biocatalysts via Protein Design: The Case of Lipases, FEMS Microbiol. Rev., 16, 253–257.
Delagrave, S., Goldman, E.R., and Youvan, D.C. (1993) Recursive Ensemble Mutagenesis, Protein Eng. 6, 327–331.
Chen, K., and Arnold, F.H. (1993) Tuning the Activity of an Enzyme for Unusual Environments: Sequential Random Mutagenesis of Subtilisin E for Catalysis in Dimethylformamide, Proc. Natl. Acad. Sci. USA 90, 5618–5622.
Stemmer, W.P.C. (1995) Searching Sequence Space, Biotechnology, 13, 549–553.
Author information
Authors and Affiliations
About this article
Cite this article
Klein, R.R., King, G., Moreau, R.A. et al. Altered acyl chain length specificity of Rhizopus delemar lipase through mutagenesis and molecular modeling. Lipids 32, 123–130 (1997). https://doi.org/10.1007/s11745-997-0016-1
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-997-0016-1