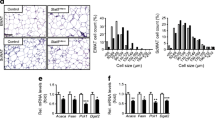
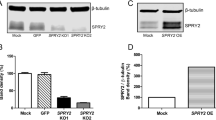
Abstract
Berardinelli-Seip congenital lipodystrophy (BSCL) is an autosomal recessive disorder. The more severe form, designated BSCL2, arises due to mutations in the BSCL2 gene. Patients with BSCL2, as well as Bscl2 −/− mice, have a near total absence of body fat, an organomegaly, and develop metabolic disorders including insulin resistance and hepatic steatosis. The function of the Seipin (BSCL2) protein remains poorly understood. Several lines of evidence have indicated that Seipin may have distinct functions in adipose versus non-adipose cells. Here we present evidence that BSCL2/Bscl2 plays a role in lipid droplet (LD) biogenesis and homeostasis in primary and cultured hepatocytes. Our results show that decreasing BSCL2/Bscl2 expression in hepatocytes increases the number and size of LD, as well as the expression of genes implicated in their formation and stability. We also show that knocking down SCD1 expression reverses the phenotype associated with Seipin deficiency. Interestingly, BSCL2 knockdown induces SCD1 expression and activity, potentially leading to increased basal phosphorylation of proteins involved in the insulin signaling cascade, as well as further increasing fatty acid uptake and de novo lipogenesis. In conclusion, our results suggest that a hepatic BSCL2/Bscl2 deficiency induces the increase and expansion of LD, potentially via increased SCD1 activity.










Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AGPAT:
-
1-Acylglycerol-3-phosphate O-acyltransferases
- ATGL:
-
Adipose triglyceride lipase
- ATF6:
-
Activating transcription factor-6
- ANOVA:
-
Analysis of variance
- BSCL:
-
Berardinelli-Seip congenital lipodystrophy
- BSA:
-
Bovine serum albumin
- CIDEA:
-
Cell death-inducing DFFA-like effector a
- CE:
-
Cholesteryl esters
- DAG:
-
Diacylglycerol
- PERK:
-
Double-stranded RNA-dependent protein kinase-like ER kinase
- ER:
-
Endoplasmic reticulum
- FAS:
-
Fatty acid synthase
- FRQNT:
-
Fond de Recherche du Québec-Nature et Technologie
- G6pc:
-
Glucose-6-phosphatase catalytic subunit
- Gck:
-
Glucokinase
- GRP78:
-
Glucose-regulated protein 78
- GTT:
-
Glucose tolerance test
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HPRT1:
-
Hypoxanthine phosphoribosyltransferase 1
- IRE1a:
-
Inositol-requiring transmembrane kinase and endonuclease 1a
- IRS:
-
Insulin receptor substrate
- ITT:
-
Insulin tolerance test
- KO:
-
Knockout
- LD:
-
Lipid droplet
- mTOR:
-
Mechanistic target of rapamycin
- MUFA:
-
Monounsaturated fatty acids
- NSERC:
-
National Science and Engineering Research Council of Canada
- NASH:
-
Non-alcoholic steatohepatitis
- PPAR:
-
Peroxisome proliferator activated receptor
- PLIN5:
-
Perilipin 5
- Pepck:
-
Phosphoenolpyruvate carboxykinase
- PBS:
-
Phosphate-buffered saline
- PL:
-
Phospholipids
- SFA:
-
Saturated fatty acids
- SREBP-1c:
-
Sterol regulatory element binding protein-1c
- SCD1:
-
Stearoyl-CoA desaturase 1
- TAG:
-
Triacylglycerol
- FFA:
-
Unesterified fatty acids
- UPR:
-
Unfolded protein response
- WT:
-
Wildtype
References
Garg A (2011) Clinical review#: lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab 96(11):3313–3325
Berardinelli W (1954) An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab 14(2):193–204
Seip M (1959) Lipodystrophy and gigantism with associated endocrine manifestations. A new diencephalic syndrome? Acta Paediatr 48:555–574
Agarwal AK, Garg A (2004) Seipin: a mysterious protein. Trends Mol Med 10(9):440–444
Magre J et al (2001) Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28(4):365–370
Agarwal AK, Garg A (2003) Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab 14(5):214–221
Cui X et al (2011) Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet 20(15):3022–3030
Prieur X et al (2013) Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia 56(8):1813–1825
Chen W et al (2012) Berardinelli-seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol Cell Biol 32(6):1099–1111
Payne VA et al (2008) The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes 57(8):2055–2060
Liu L et al (2014) Adipose-specific knockout of SEIPIN/BSCL2 results in progressive lipodystrophy. Diabetes 63(7):2320–2331
Unger RH (2002) Lipotoxic diseases. Annu Rev Med 53:319–336
van Herpen NA, Schrauwen-Hinderling VB (2008) Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav 94(2):231–241
Szendroedi J, Roden M (2009) Ectopic lipids and organ function. Curr Opin Lipidol 20(1):50–56
Gao M et al (2015) Expression of seipin in adipose tissue rescues lipodystrophy, hepatic steatosis and insulin resistance in seipin null mice. Biochem Biophys Res Commun 460(2):143–150
Chen W et al (2014) Molecular mechanisms underlying fasting modulated liver insulin sensitivity and metabolism in male lipodystrophic Bscl2/Seipin-deficient mice. Endocrinology 155(11):4215–4225
Cartwright BR, Goodman JM (2012) Seipin: from human disease to molecular mechanism. J Lipid Res 53(6):1042–1055
Fei W, Du X, Yang H (2011) Seipin, adipogenesis and lipid droplets. Trends Endocrinol Metab 22(6):204–210
Fei W et al (2008) Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol 180(3):473–482
Wolinski H et al (2011) A role for seipin in lipid droplet dynamics and inheritance in yeast. J Cell Sci 124(Pt 22):3894–3904
Wolinski H et al (2015) Seipin is involved in the regulation of phosphatidic acid metabolism at a subdomain of the nuclear envelope in yeast. Biochim Biophys Acta 1851(11):1450–1464
Wang CW, Miao YH, Chang YS (2014) Control of lipid droplet size in budding yeast requires the collaboration between Fld1 and Ldb16. J Cell Sci 127(Pt 6):1214–1228
Fei W et al (2011) A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet 7(7):e1002201
Szymanski KM et al (2007) The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci USA 104(52):20890–20895
Fei W et al (2011) Molecular characterization of seipin and its mutants: implications for seipin in triacylglycerol synthesis. J Lipid Res 52(12):2136–2147
Tian Y et al (2011) Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet 7(4):e1001364
Yang W et al (2013) Seipin differentially regulates lipogenesis and adipogenesis through a conserved core sequence and an evolutionarily acquired C-terminus. Biochem J 452(1):37–44
Harris CA et al (2011) DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res 52(4):657–667
Brasaemle DL, Wolins NE (2012) Packaging of fat: an evolving model of lipid droplet assembly and expansion. J Biol Chem 287(4):2273–2279
Talukder MM et al (2015) Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis. Mol Metab 4(3):199–209
Yang W et al (2014) BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum Mol Genet 23(2):502–513
Bi J et al (2014) Seipin promotes adipose tissue fat storage through the ER Ca(2)(+)-ATPase SERCA. Cell Metab 19(5):861–871
Puri P et al (2008) Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134(2):568–576
Puri V et al (2007) Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem 282(47):34213–34218
Fang DL et al (2013) Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells. Mol Cell Biochem 381(1–2):127–137
Rinella ME et al (2011) Dysregulation of the unfolded protein response in db/db mice with diet-induced steatohepatitis. Hepatology 54(5):1600–1609
Gentile CL, Frye M, Pagliassotti MJ (2011) Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid Redox Signal 15(2):505–521
Zhang XQ et al (2014) Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 20(7):1768–1776
Zhang K et al (2011) The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J 30(7):1357–1375
Zhang C et al (2012) Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology 55(4):1070–1082
Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14(1):20–28
Harbrecht BG et al (2001) cAMP inhibits inducible nitric oxide synthase expression and NF-kappaB-binding activity in cultured rat hepatocytes. J Surg Res 99(2):258–264
Harbrecht BG et al (1996) Glucagon inhibits hepatocyte nitric oxide synthesis. Arch Surg 131(12):1266–1272
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Ralston JC et al (2014) Inhibition of stearoyl-CoA desaturase-1 in differentiating 3T3-L1 preadipocytes upregulates elongase 6 and downregulates genes affecting triacylglycerol synthesis. Int J Obes (Lond) 38(11):1449–1456
Fernandez C et al (2011) Altered desaturation and elongation of fatty acids in hormone-sensitive lipase null mice. PLoS One 6(6):e21603
Attie AD et al (2002) Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res 43(11):1899–1907
Stremmel W, Berk PD (1986) Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci USA 83(10):3086–3090
Pohl J, Ring A, Stremmel W (2002) Uptake of long-chain fatty acids in HepG2 cells involves caveolae: analysis of a novel pathway. J Lipid Res 43(9):1390–1399
Bolker HI et al (1956) The incorporation of acetate-1-C14 into cholesterol and fatty acids by surviving tissues of normal and scorbutic guinea pigs. J Exp Med 103(2):199–205
Jin FY, Kamanna VS, Kashyap ML (1999) Niacin accelerates intracellular ApoB degradation by inhibiting triacylglycerol synthesis in human hepatoblastoma (HepG2) cells. Arterioscler Thromb Vasc Biol 19(4):1051–1059
Jiang M et al (2014) Lack of testicular seipin causes teratozoospermia syndrome in men. Proc Natl Acad Sci USA 111(19):7054–7059
Wu L et al (2014) Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci China Life Sci 57(1):107–116
Wang H et al (2011) Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res 52(12):2159–2168
Beller M et al (2008) COPI complex is a regulator of lipid homeostasis. PLoS Biol 6(11):e292
Guo Y et al (2008) Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453(7195):657–661
Soni KG et al (2009) Coatomer-dependent protein delivery to lipid droplets. J Cell Sci 122(Pt 11):1834–1841
He J et al (2011) The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med (Maywood) 236(10):1116–1121
Cheon Y et al (2005) Induction of overlapping genes by fasting and a peroxisome proliferator in pigs: evidence of functional PPARalpha in nonproliferating species. Am J Physiol Regul Integr Comp Physiol 288(6):R1525–R1535
Louet JF et al (2001) Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem J 354(Pt 1):189–197
Berardinelli SD, Fischer RM, Katz I (1956) Congenital absence of the pectoral muscle. Am J Roentgenol Radium Ther Nucl Med 76(3):599–604
Thomas SE et al (2010) Diabetes as a disease of endoplasmic reticulum stress. Diabetes Metab Res Rev 26(8):611–621
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110(10):1389–1398
Lee JS et al (2012) Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett 211(1):29–38
Chen W et al (2009) The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology 150(10):4552–4561
Kim MS et al (2014) A draft map of the human proteome. Nature 509(7502):575–581
Lundin C et al (2006) Membrane topology of the human seipin protein. FEBS Lett 580(9):2281–2284
Ntambi JM et al (2002) Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99(17):11482–11486
Walther TC, Farese RV Jr (2009) The life of lipid droplets. Biochim Biophys Acta 1791(6):459–466
Novikoff AB et al (1980) Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol 87(1):180–196
Perktold A et al (2007) Organelle association visualized by three-dimensional ultrastructural imaging of the yeast cell. FEMS Yeast Res 7(4):629–638
Ito D, Suzuki N (2009) Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain 132(Pt 1):8–15
Yagi T et al (2011) N88S seipin mutant transgenic mice develop features of seipinopathy/BSCL2-related motor neuron disease via endoplasmic reticulum stress. Hum Mol Genet 20(19):3831–3840
Holtta-Vuori M et al (2013) Alleviation of seipinopathy-related ER stress by triglyceride storage. Hum Mol Genet 22(6):1157–1166
Shi X et al (2013) Regulation of lipid droplet size and phospholipid composition by stearoyl-CoA desaturase. J Lipid Res 54(9):2504–2514
Arisawa K et al (2013) Changes in the phospholipid fatty acid composition of the lipid droplet during the differentiation of 3T3-L1 adipocytes. J Biochem 154(3):281–289
Miyazaki M et al (2007) Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab 6(6):484–496
Man WC et al (2006) Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res 47(9):1928–1939
Kim E et al (2011) Inhibition of stearoyl-CoA desaturase1 activates AMPK and exhibits beneficial lipid metabolic effects in vitro. Eur J Pharmacol 672(1–3):38–44
Chen W et al (2014) Molecular mechanisms underlying fasting modulated liver insulin sensitivity and metabolism in male lipodystrophic Bscl2/Seipin-deficient mice. Endocrinology p. en20141292
Coll T et al (2008) Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283(17):11107–11116
Nardi F et al (2014) Enhanced insulin sensitivity associated with provision of mono and polyunsaturated fatty acids in skeletal muscle cells involves counter modulation of PP2A. PLoS One 9(3):e92255
Xiao C et al (2006) Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 49(6):1371–1379
Vessby B et al (2001) Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 44(3):312–319
Salvado L et al (2013) Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 56(6):1372–1382
O’Brien RM, Granner DK (1996) Regulation of gene expression by insulin. Physiol Rev 76(4):1109–1161
Mendez-Lucas A et al (2014) Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability. J Biol Chem 289(32):22090–22102
Foufelle F, Ferre P (2002) New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J 366(Pt 2):377–391
Foretz M et al (1998) AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem 273(24):14767–14771
Zhou G et al (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108(8):1167–1174
Decaux JF, Antoine B, Kahn A (1989) Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem 264(20):11584–11590
Prip-Buus C et al (1995) Induction of fatty-acid-synthase gene expression by glucose in primary culture of rat hepatocytes. Dependency upon glucokinase activity. Eur J Biochem 230(1):309–315
O’Callaghan BL et al (2001) Glucose regulation of the acetyl-CoA carboxylase promoter PI in rat hepatocytes. J Biol Chem 276(19):16033–16039
Koo SH, Dutcher AK, Towle HC (2001) Glucose and insulin function through two distinct transcription factors to stimulate expression of lipogenic enzyme genes in liver. J Biol Chem 276(12):9437–9445
Waters KM, Ntambi JM (1994) Insulin and dietary fructose induce stearoyl-CoA desaturase 1 gene expression of diabetic mice. J Biol Chem 269(44):27773–27777
Foufelle F et al (1992) Glucose stimulation of lipogenic enzyme gene expression in cultured white adipose tissue. A role for glucose 6-phosphate. J Biol Chem 267(29):20543–20546
Jones BH et al (1998) Glucose induces expression of stearoyl-CoA desaturase in 3T3-L1 adipocytes. Biochem J 335(Pt 2):405–408
Acknowledgements
We wish to thank Dr. Jocelyne Magre (University of Nantes, France) for kindly providing us with mRNA and protein extracts from Bscl2 −/− mice, as well as Dr. Xiaoqin Ye (University of Georgia, USA) for samples of Bscl2 −/− mouse liver. We also thank Denis Flipo for his precious help with confocal microscopy, the laboratory of Dr. Diana Averill for primary rat hepatocytes, and Dr. Daniel Boismenu for his help with data analysis and discussion. The Discovery Grants Program of the National Science and Engineering Research Council of Canada (NSERC) funded this research. MAL and SL were supported by the Fond de Recherche du Québec-Nature et Technologie (FRQNT) fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
About this article
Cite this article
Lounis, M.A., Lalonde, S., Rial, S.A. et al. Hepatic BSCL2 (Seipin) Deficiency Disrupts Lipid Droplet Homeostasis and Increases Lipid Metabolism via SCD1 Activity. Lipids 52, 129–150 (2017). https://doi.org/10.1007/s11745-016-4210-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4210-5