Abstract
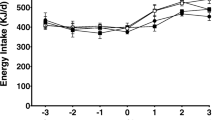
Dietary trans-fatty acids (t-FA) during pregnancy have adverse effects on growth and development. To determine the effect of dietary t-FA during just the first half of pregnancy, rats were given a diet containing 8 % hydrogenated peanut oil and 2 % olive oil (PO) and compared to rats given a diet containing 10 % olive oil (OO). After 12 days all rats were fed standard diet and were studied at days 12 or 20 of pregnancy or days 1 or 6 postpartum. At day 12 of pregnancy there were small differences in the plasma and lumbar adipose tissue fatty acid profiles and elaidic acid [18:1(n-9)t] was present in the PO group. From day 12 to 20 of pregnancy, plasma non-esterified fatty acids, glycerol, triacylglycerols (TAG) and most individual fatty acids increased more in PO than in OO. At day 20 of pregnancy in the PO group most plasma elaidic acid appeared as plasma TAG and was also present in the mammary gland, to decline in both sites at day 1 postpartum. Elaidic acid concentration was low in the plasma of 20-day fetuses, increased in 1-day newborns declining at day 6. Thus t-FA, eaten during early pregnancy, accumulated in maternal adipose tissue and were released during late pregnancy to be taken up by the mammary gland becoming available to the newborns during suckling.

Similar content being viewed by others
Abbreviations
- d12PR:
-
Day 12 of pregnancy
- d20PR:
-
Day 20 of pregnancy
- d1PP:
-
Day 1 postpartum
- d6PP:
-
Day 6 postpartum
- LC-PUFA:
-
Long-chain polyunsaturated fatty acids
- LPL:
-
Lipoprotein lipase
- NEFA:
-
Non-esterified fatty acids
- N.D.:
-
Not detected
- OO:
-
Olive oil diet group
- PO:
-
Hydrogenated peanut oil diet group
- SEM:
-
Standard error of the mean
- TAG:
-
Triacylglycerols
- t-FA:
-
trans-Fatty acids
- VLDL:
-
Very low density lipoproteins
References
Craig-Schmidt MC (1998) Worldwide consumption of trans fatty acids. In: Sebedio JL, Christie WW (eds) Trans fatty acids in human nutrition. The Oily Press, Dundee, pp 59–113
Elias SL, Innis SM (2002) Bakery foods provide the major sources of trans fatty acids among pregnant women with diets providing 30 % energy from fat. Am J Diet Assoc 102:46–51
Van Poppel G, On behalf of the TRANSFair study group (1998) Intake of trans fatty acids in western Europe: the TRANSFAIR STUDY. Lancet 351:1099
Clifton PM, Keogh JB, Noakes M (2004) Trans fatty acids in adipose tissue and the food supply are associated with myocardial infarction. J Nutr 134:874–879
Baylin A, Kabagambe EK, Siles X, Campos H (2002) Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 76:750–757
Larqué E, Zamora S, Gil A (2000) Dietary trans fatty acids affect the essential fatty-acid concentration of rat milk. J Nutr 130:847–851
Ross AC, Davila ME, Cleary MP (1985) Fatty acids and retinyl esters of rat milk: effects of diet and duration of lactation. J Nutr 115:1488–1497
Carlson SE, Clandinin MT, Cook HW, Emken EA, Filer LJ Jr (1997) trans fatty acids: infant and fetal development. Am J Clin Nutr 66:717S–736S
Herrera E, Ramos MP (2008) Long-term effects of trans fatty acid intake during pregnancy and lactation: does it has deleterious consequences? Futur Lipidol 3:489–494
Innis SM (2006) Trans fatty acids during pregnancy, infancy and early childhood. Atheroscler Suppl 7:17–20
Guimaräes DED, de Castro J, Martinez-Botas J, Sardinha FLC, Ramos P, Herrera E, Tavares do Carmo MG (2009) Early and prolonged intake of partially hydrogenated fat alters the expression of genes in rat adipose tissue. Nutrition 25:782–789
Pisani LP, Oller do Nascimento CM, Bueno AA, Biz C, Albuquerque KT, Ribeiro EB, Oyama LM (2008) Hydrogenated fat diet intake during pregnancy and lactation modifies the PAI-I gene expression in white adipose tissue of offspring in adult life. Lipids Health Dis 7:12
Pettersen J, Opstvedt J (1989) Fatty acid composition of the brain and other organs in the newborn piglet. Lipids 24:616–624
Johnston PV, Johnson OC, Kummerow FA (1957) Non transfer of trans fatty acids from other to young. Proc Soc Exp Biol Med 99:760–762
Johnston PV, Kummerow FA, Walton CH (1958) Origin of the trans fatty acids in human tissue. Proc Soc Exp Biol Med 99:735–736
Koletzko B, Müller J (1990) Cis- and trans-isomeric fatty acids in plasma lipids of newborn infants and their mothers. Biol Neonate 57:172–178
Houwelingen AC, Hornstra G (1994) Trans fatty acids in early human development. World Rev Nutr Diet 75:175–178
Fernandes FS, Tavares do Carmo M, Herrera E (2012) Influence of maternal diet during early pregnancy on the fatty acid profile in the fetus at late pregnancy in rats. Lipids 47:505–517
Sardinha FLC, Fernandes FS, Tavares do Carmo M, Herrera E (2012) Sex-dependent nutritional programming: fish oil intake during early pregnancy in rats reduces age-dependent insulin resistance in male, but not female, offspring. Am J Physiol Regul Integr Comp Physiol 304:R313–R320
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 22:24–36
Amusquivar E, Schiffner S, Herrera E (2011) Evaluation of two methods for plasma fatty acid analysis gy GC. Eur J Lipid Sci Technol 113:711–716
Mead JR, Irvine SA, Ramji DP (2002) Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med 80:753–769
Wajchenberg BL, Glannella-Neto D, da Silva MER, Santos RF (2002) Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res 34:616–621
Casteilla L, Pénicaud L, Cousin B, Calise D (2008) Choosing an adipose tissue depot for sampling: factors in selection and depot specificity. In: Yang K (ed) Adipose tissue protocols. Hunmana Press, Totowa, pp 23–38
Moore CE, Alfin-Slater RB, Aftergood L (1980) Incorporation and disappearance of trans-fatty acids in rat tissues. Am J Clin Nutr 33:2318–2323
Hanis T, Zidk V, Sachova J, Klir P, Deyl Z (1989) Effects of dietary trans-fatty acids on reproductive performance of Wistar rats. Br J Nutr 61:519–529
Ramos MP, Crespo-Solans MD, del Campo S, Herrera E (2003) Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am J Physiol Endocrinol Metab 285:E318–E328
Knopp RH, Herrera E, Freinkel N (1970) Carbohydrate metabolism in pregnancy. VIII. Metabolism of adipose tissue isolated from fed and fasted pregnant rats during late gestation. J Clin Invest 49:1438–1446
Chaves JM, Herrera E (1980) In vitro response of glycerol metabolism to insulin and adrenalin in adipose tissue from fed and fasted rats during pregnancy. Biol Neonate 38:139–145
Saravanan N, Haseeb A, Ehtesham NZ, Ghafoorunissa IA (2005) Differential effects of dietary saturated and trans-fatty acids on expression of genes associated with insulin sensitivity in rat adipose tissue. Eur J Endocrinol 153:159–165
Ramirez I, Llobera M, Herrera E (1983) Circulating triacylglycerols, lipoproteins, and tissue lipoprotein lipase activities in rat mothers and offspring during the perinatal period: effect of postmaturity. Metabolism 32:333–341
Argiles J, Herrera E (1989) Appearance of circulating and tissue 14C-lipids after oral 14C-tripalmitate administration in the late pregnant rat. Metabolism 38:104–108
Pereira Assumpçäo R, Duarte dos Santos F, de MattosMachadoAndrade P, Freire Barreto G, Tavares do Carmo MG (2004) Effect of variation of trans-fatty acid in lactating rats diet on lipoprotein lipase activity in mammary gland, liver, and adipose tissue. Nutrition 20:806–811
Koletzko B (1992) Trans fatty acids may impair biosynthesis of long chain polyunsaturates and growth in man. Acta Paediatr 81:302–306
Elias SL, Innis SM (2001) Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr 73:807–814
Hornstra G (2000) Essential fatty acids in mothers and their neonates. Am J Clin Nutr 71(suppl):1262S–1269S
Craig-Smidt MC (2001) Isomeric fatty acids: evaluating status and implications for maternal and child health. Lipids 36:997–1006
Assumpçäo RP, Santos FD, Setta CL, Barreto GF, Mata IEA, Estadella D, Azeredo VB, Tavares do Carmo MG (2002) Trans-fatty acids in maternal diet may impair lipid biosynthesis in mammary gland of lactating rats. Ann Nutr Metab 46:169–175
Amusquivar E, Laws J, Clark L, Herrera E (2010) Fatty acid composition of the maternal diet during the first or the second half of gestation influences the fatty acid composition of sows’ milk and plasma of their piglets. Lipids 45:409–418
Acknowledgments
We thank Milagros Morante for her excellent technical assistance and pp-science-editing.com for editing and linguistic revision of the manuscript. This work was carried out with the financial support of Fundación Ramón Areces of Spain (Grant CIVP16A1835).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Amusquivar, E., Sánchez-Blanco, C., Clayton, J. et al. Maternal Consumption of trans-Fatty Acids During the First Half of Gestation are Metabolically Available to Suckled Newborn Rats. Lipids 49, 265–273 (2014). https://doi.org/10.1007/s11745-014-3879-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-014-3879-6