Abstract
The impact of a moderate Zn deficiency on growth and plasma and liver lipids was investigated in two 4-week experiments with male weanling rats fed fat-enriched diets. Semisynthetic, approximately isocaloric diets containing 3% soybean oil were supplemented with either 7 or 100 mg Zn/kg diet and with 22% beef tallow (BT) or sunflower oil (SF). In Experiment 1, which compared the dietary fat level and the fat source in a factorial design of treatments, all diets were fed ad libitum to 6 × 8 animals, whereas intake of the high-Zn BT and SF diets was restricted in Experiment 2 (5 × 6 rats) to the level of intake of the respective low-Zn diets. The low-Zn SF diet consistently depressed food intake and final live weights of the animals to a greater extent than the other low-Zn diets, while intake and growth were comparable among the animals fed the high-Zn diets. The marginal Zn deficit per se did not alter plasma triglyceride and cholesterol concentrations nor hepatic concentrations of triglyceride, cholesterol and phospholipids. The fatty acid pattern of liver phospholipids did not indicate that chain elongation and desaturation of fatty acids was impaired by a lack of zinc. It was concluded that dietary energy and fat intake, and fat source have a greater effect on plasma and liver lipids than a moderate Zn deficiency. Marginally Zn-deficient diets enriched with sunflower oil as a major energy source cause a greater growth retardation than diets rich in carbohydrates or beef tallow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In young animals, early signs of severe zinc (Zn) deficiency are growth retardation, depressed food intake and a cyclic feeding pattern [1–3]. The dietary fat source and fat level may alter the effects of dietary Zn depletion on growth rate and lipid metabolism. Bettger et al. [4] found that dietary substitution of 5% hydrogenated coconut oil for corn oil as a source of essential fatty acids improved the growth of young rats, suggesting an interaction between these nutrients. In similar studies with chicks [5], the effects were the opposite. The addition of corn oil, soybean oil or a mixture of polyunsaturated fatty acids (PUFA) to Zn-deficient diets retarded growth of the animals to a greater extent as compared to hydrogenated coconut oil and exacerbated the symptoms of Zn deficiency. Huang et al. [6] reported that growth retardation of young rats was partially alleviated when the animals were given daily subcutaneous injections of primrose oil, but when safflower oil was injected the effect was minimal. In a recent study [7], fat source in fat-enriched diets markedly affected growth rates of weanling rats when the dietary Zn supply was marginal, i.e. about two-third of the minimum requirement for unlimited growth. Low-Zn diets supplemented with 18% sunflower oil or olive oil depressed final live weights of the animals to a greater extent than diets enriched with beef tallow, whereas no difference was evident among corresponding high-Zn diets.
The objective of the present study was to investigate the impact of highly fat-enriched diets on growth and lipid metabolism of weanling rats when dietary Zn supply is either marginal or adequate for unlimited growth. Semisynthetic diets were supplemented with 7 or 100 μg Zn/g and were enriched with 22% fat as beef tallow (BT) or as sunflower oil (SF) above a basal fat content of 3% soybean oil in all diets. In a first experiment, which included diets without supplemental fat, all diets were fed ad libitum for 4 weeks, whereas intake of the high-Zn diets was restricted in a second experiment according to the intake of the respective low-Zn BT and SF diets. The low-Zn SF diet consistently reduced food and energy intake and final live weights to a greater extent than the other low-Zn diets. Changes in plasma and liver lipids observed in response to dietary treatments were primarily attributable to differences in energy and fat intake and to fat source rather than to the moderate Zn deficiency. We conclude that dietary energy and fat intake, and fat source have a greater effect on plasma and liver lipids than a moderate Zn deficit, and that Zn-deficient diets enriched with sunflower oil as a major energy source cause a greater growth retardation than diets rich in carbohydrates or beef tallow.
Experimental Procedures
Animals and Diets
In Experiment 1, a total of 48 male weanling Wistar rats (Harlan Winkelmann, Borchen, Germany) with an initial live weight of 48 ± 2.8 g (mean ± SD) were randomly assigned to six treatment groups of eight animals each, following a 2 × 3 factorial design for dietary Zn and fat treatments. Semisynthetic diets for these groups differed in Zn supplementation (7 vs. 100 μg/g, added as ZnSO4·7H2O), and in fat supplementation: 22% beef tallow (BT) or 22% sunflower oil (SF) versus a low-fat, carbohydrate-rich control diet (CT). All diets contained 3% soybean oil to offer sufficient amounts of essential fatty acids. The major components of the diets are summarized in Table 1. They contributed a basal Zn level of about 0.5 μg/g by analysis. In the high-fat diets parts of cornstarch were replaced by cellulose to compensate for differences in energy. The fatty acid composition of the low- and high-fat diets is shown in Table 2. Diets were stored at 4 °C. Ad libitum feeding was practiced, replacing remainders by fresh portions daily.
In Experiment 2, six male weanling rats each (50 ± 2.8 g initial live weight) were randomly allocated to five dietary treatments: (1) low-Zn BT diet fed ad libitum, (2) high-Zn BT diet fed in restricted amounts according to average intake of group 1, (3) low-Zn SF diet fed ad libitum, (4) high-Zn SF diet fed in restricted amounts according to average intake of group 3, and (5) high-Zn SF diet fed ad libitum. Diet composition was the same as described in Tables 1 and 2 regarding ingredients, Zn and fat supplementation. Food consumption was determined daily to control intake of the rats in group 2 and 4.
Approval of the experimental protocols for use and care of laboratory animals in research was obtained by the regional Animal Protection Authority (Regierungspräsidium Giessen, II 25.3-19c20/15c GI 19/3). The rats were housed individually in stainless steel metabolic cages in an environmentally controlled room (22 °C, rel. humidity of 55%, 12 h light and dark cycle). They had free access to deionized water. In Experiment 1, feces were collected daily during week 2 and 3, freeze-dried and stored at 4 °C for determination of energy and fat content. After 4 weeks, the animals were fasted overnight and anesthetized with carbon dioxide followed by decapitation. Blood was collected in test tubes containing heparin and centrifuged (3,000g for 10 min at 4 °C), and the resulting plasma was stored at −80 °C. The liver and right femur were excised, weighed and frozen on dry ice for subsequent measurements.
Analytical Procedures
Diets were analyzed for dry matter, crude protein, fat and neutral detergent fiber by official methods [9]. Fecal samples were treated with 4 M HCl before fat extraction using n-hexane. Gross energy (GE) in diets and feces was determined by adiabatic bomb calorimetry. The metabolizable energy (ME) of the low- and high-fat diets was calculated as digestible energy (intake minus fecal excretion of GE during week 2 and 3 of Experiment 1) multiplied by 0.96 [10].
Zn concentration in the femur bone and diets was analyzed by ICP-AES (inductively coupled plasma atomic emission spectrometry) after dry-ashing (450 °C) and dissolving in 3 M HNO3. Plasma Zn concentration was measured directly after dilution with 0.1 M hydrochloric acid by flame atomic absorption spectrometry. The activity of the alkaline phosphatase (AP; EC 3.1.3.1) in plasma was determined photometrically with a standard method [11]. The activity of the glucose-6-phosphate dehydrogenase (G6PD; EC 1.1.1.49) in liver homogenates was assayed with a commercial reagent kit (Boehringer Mannheim GmbH, Germany). The protein content in liver homogenates was determined by the Lowry method [12].
Liver total lipids were extracted with a mixture of hexane:isopropanol (3:2, by vol) according to the method of Hara and Radin [13] with minor modifications. Briefly, frozen liver was weighed and homogenized in 0.15 M NaCl solution. Hexane:isopropanol (3:2, by vol), containing 0.005% (wt/vol) butylhydroxytoluol, was added and the mixture centrifuged for 10 min at 3,000g and 10 °C. The upper organic phase was collected and the solvent evaporated under liquid nitrogen. The resulting residues were quantified gravimetrically as total lipids and resuspended in hexane:isopropanol (3:2, by vol). Aliquots of the liver lipid extracts and plasma samples were analyzed for their content of triglycerides and cholesterol using commercial test kits (Ecoline 25, Merck KGaA, Darmstadt, Germany). The phospholipid (PL) content was quantified by phosphate measurement in aliquots of the liver lipid extracts using a commercial test combination for phosphorus and phospholipids (Boehringer Mannheim GmbH, Germany).
Liver PL were separated from the total lipid extracts for subsequent fatty acid analysis by aminopropyl-bonded phase columns (Bond Elut, Agilent Technologies); this solid phase extraction method has been shown to be superior to other separation techniques [14]. Aliquots of liver lipid extracts were dissolved in chloroform and applied to hexane pre-conditioned columns. After treatment with several other solvents that released neutral lipids and free fatty acids from the column, methanol was added to provoke the elution of the PL fraction. These fractions, to which heptadecanoic acid (17:0) was added as internal standard, were subjected to transmethylation with N-trimethyl sulfonium hydroxide. Fatty acid methyl esters (FAME) were separated by gas chromatography. They were identified by comparing retention times of FAME in a standard mix (Supelco 37, Sigma-Aldrich, Inc) that was supplemented with docosapentaenoic acid (D5679, Sigma-Aldrich, Inc). Individual FA were quantified on the basis of area under the curve and internal standard response as molar percentage of the total amount of fatty acids.
Statistical Analyses
Statistical analyses were performed using IBM SPSS Statistics 19 for Windows (IBM Company, Chicago, IL, USA). Data were analyzed by two-way analysis of variance (ANOVA) according to the linear model for a completely randomized design to test for treatment effects of Zn, fat and Zn × fat interaction (2 × 3-factorial in Expt. 1 and 2 × 2-factorial in Expt. 2). Since the factor “fat” included fat level (low vs. high) and fat source (BT vs. SF) in Experiment 1, bifactorial ANOVA was also applied to the high-fat treatments to test for effects of fat source. In addition, univariate analysis was applied to test for differences among groups within each dietary Zn level in Experiment 1 and among the three groups fed the SF diets in Experiment 2. The Tukey test was used as a post hoc test to compare treatment means or, alternatively, the Games–Howell test if homogeneity of variance (Levene test) could not be attained by logarithmic (ln) or square root transformation of the data. The significance was set at p < 0.05 in post hoc tests.
Results
Experiment 1
Food and Energy Intake and Final Live Weight
In Experiment 1, in which all diets were offered ad libitum during the 28-days feeding period, the marginal Zn supplementation of the diets markedly (p < 0.001) reduced food and energy intake and final live weights (Table 3). ME intake and final live weights significantly differed among the rats fed the low-Zn diets, but not among the animals fed the high-Zn diets. Both level and source of dietary fat contributed to a significant Zn × fat interaction, as the reductions in ME intake and final live weights due to the moderate Zn deficiency differed among the CT, BT and SF groups (about 14, 22 and 30% in ME intake and 12, 19 and 28% in final weights relative to the respective high-Zn groups). ME intake per gram of weight gain was significantly higher for the rats fed the low-Zn diets, but the difference was relatively small (less than 7%), and may be attributed primarily to a higher proportion of energy expended for maintenance in the animals fed the low-Zn diets.
Fat and Energy Digestibility
Fat digestibility in the BT groups was approximately 8–9% U lower than in the CT and SF groups independent of the dietary Zn level (Table 3). This difference may be attributed to the high content of saturated fatty acids in beef tallow. Gross energy (GE) digestibility of the BT and SF diets was markedly lower than that of the CT diets (about 20 and 15% U, respectively; p < 0.001), reflecting the pronounced difference in dietary cellulose content. GE digestibility also significantly (p < 0.001) differed between the BT and SF diets according to the difference in fat digestibility.
Zinc and Metabolic Status
Zn concentrations in plasma and femur and activity of the alkaline phosphatase (AP) in plasma, which were analyzed as indicators of Zn status, were substantially reduced (p < 0.001) by the marginal dietary Zn supply in Experiment 1 (Table 3). Dietary fat level and fat source also affected these parameters to different degrees. Plasma Zn concentrations were lower in the rats fed the high-fat diets as compared to those fed the CT diets. This difference was most marked for the SF-fed animals. The plasma AP activity was enhanced by the high-fat diets.
The activity of glucose-6-phosphate dehydrogenase (G6PD), a member of the lipogenic enzyme family [15], was greatly reduced (p < 0.001) in the liver of the rats fed the high-fat diets as compared to those given the low-fat CT diet, regardless of the dietary Zn level (Table 3).
Plasma and Liver Lipids
Plasma concentrations of triglycerides (TAG), total cholesterol (TC) and HDL-C were not significantly altered by the dietary Zn level, but they were by the level and type of fat in the diets in Experiment 1 (Table 4). The SF diets consistently reduced plasma TAG concentrations regardless of the dietary Zn level. TC and HDL-C concentrations in the low-Zn CT group were significantly higher (p < 0.05) than in the low-Zn BT and SF groups, but not when the dietary Zn level was high. Fat source did not significantly affect plasma TC and HDL-C concentrations.
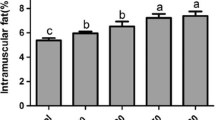
Liver concentrations of total lipids, TAG and cholesterol (Table 4) were significantly higher in the rats fed the high-Zn BT and SF diets than in those fed the respective low-Zn diets, while they did not differ between the two CT-fed groups. Concentrations of these lipids were substantially higher for the SF-fed than for the BT-fed animals (p < 0.001). In contrast, hepatic PL concentrations were not altered by any of the dietary treatments.
Fatty Acid Composition of Liver Phospholipids
Table 5 presents the fatty acid pattern in the PL extracted from the liver in Experiment 1. Total molar proportions of saturated fatty acids (SFA) were comparable across dietary treatments. However, the percentages of 16:0 and 18:0 significantly differed among groups (p < 0.001). Palmitic acid (16:0) was the major SFA in the CT groups, whereas 18:0 dominated in the BT and SF groups. Oleic acid (18:1) was the predominant monounsaturated fatty acid (MUFA) in all groups. The highest mean percentage of this fatty acid was recorded in the BT groups. As expected, the SF diets increased the total percentages of n-6 PUFA, especially those of 18:2, 20:4 and 22:4, and depressed the percentages of n-3 PUFA, leading to substantially higher ratios of n-6 PUFA to n-3 PUFA in the SF-fed groups. Significant Zn effects were noted in the case of linoleic (18:2), γ-linolenic (18:3n-6) and docosapentaenoic acid (22:5n-3), which were present in higher proportions when the rats were fed the low-Zn BT and SF diets as compared to the respective high-Zn diets. The highly significant Zn × fat interaction observed for 18:2 (p = 0.001) is basically due to the different direction and height of response to the dietary fat level among the corresponding low- and high-Zn groups. This diet effect on 18:2 is also reflected in a significant Zn × fat interaction of the n-6 PUFA to n-3 PUFA ratio (p < 0.05).
Experiment 2
Energy Intake and Final Live Weight
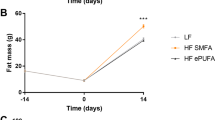
In Experiment 2, in which food intake of the high-Zn BT and SF diets was restricted to amounts consumed by the animals offered the corresponding low-Zn diets, final live weights were not affected by the difference in Zn supply, whereas the animals allowed to consume the high-Zn SF diet ad libitum reached significantly higher final live weights (Table 6). ME intake and final live weights of the rats fed the low-Zn SF diet were lower (about 13%; p < 0.01) than those fed the low-Zn BT diet. As in Experiment 1, ME intake per gram weight gain was reduced by the high-Zn diets, presumably due to a slightly leaner tissue gain.
Zinc Status
Plasma and femur Zn concentrations and plasma activity of the alkaline phosphatase (AP) were markedly reduced (p < 0.001) by the marginal dietary Zn supply in Experiment 2 (Table 6). Fat source did not significantly (p > 0.05) affect these parameters.
Plasma and Liver Lipids
Plasma concentrations of TAG, TC and HDL-C were not significantly altered by the dietary Zn level in Experiment 2 (Table 7). TAG concentrations in the SF groups were lower than those in the BT groups. But plasma TC and HDL-C concentrations were not significantly affected by fat source. The livers of the rats consuming the corresponding low- and high-Zn BT and SF-diets in equivalent quantities had comparable concentrations of total lipids, cholesterol and PL (Table 7). However, the animals fed the high-Zn SF diet ad libitum showed a significantly (p < 0.05) increased hepatic TAG content. PL concentrations were about 10% higher in the SF-fed than in the BT-fed animals.
Discussion
The present experiments were designed to investigate the effect of a moderate Zn deficiency on growth and selected features of lipid metabolism in weanling rats fed diets that greatly differed in fat level and fat source. As expected, the low-Zn diets (7 μg/g supplemental zinc) were associated with notable reductions in food and energy intake and final live weights compared to animals fed the high-Zn diets (100 μg/g supplemental zinc), but without the complications that would arise from feeding severely Zn-deficient diets, i.e. growth arrest, development of a cyclic feeding pattern, conspicuous inanition, and the manifestation of clinical deficiency symptoms [1–3]. The increase in dietary fat level implied a pronounced difference in energy intake as carbohydrate and fat in Experiment 1. Available carbohydrates (cornstarch and sucrose) and fat accounted for about 69 and 8% of ME intake of the rats fed the low-fat diets, respectively, whereas carbohydrates contributed no more than about 22% and fat at least 55% of ME intake in the case of the high-fat diets (Table 1). There was a small difference in ME content between the BT and SF diets due the fact that fat digestibility was significantly lower for the BT diets than for the SF and CT diets (Table 3).
In Experiment 1, in which all diets were fed ad libitum, mean daily ME intake and final live weights were comparable among the rats fed the three high-Zn diets regardless of dietary fat level and fat source. This, however, was not the case among the animals fed the corresponding low-Zn diets. The animals given the low-Zn SF diet displayed the greatest reductions in ME intake (about 30%) and final live weights (about 28%) relative to those consuming the corresponding high-Zn diet (Table 3). Again in Experiment 2, daily ME intake and final live weights were significantly lower for the rats fed the low-Zn SF diet as compared to the low-Zn BT diet (Table 6). This observation supports a previous study in which marginally Zn-deficient diets enriched with sunflower or olive oil depressed food intake and weight gain of young rats to a greater extent than did low-Zn diets containing equivalent percentages of beef tallow, butterfat or coconut oil [7]. Although it is a common observation that both food intake and growth rate are depressed by dietary Zn deficiency, previous studies have clearly shown that growth retardation cannot be attributed to the lower food or energy intake. Chesters and Quarterman [1] observed that force-feeding Zn-deprived rats a Zn-deficient diet not only failed to alleviate the growth arrest but rapidly induced signs of severe ill-health and morbidity. This finding has been confirmed by later investigators reporting that young rats with an initially normal Zn status cease to tolerate force-feeding of Zn-deficient diets in amounts exceeding voluntary consumption after about 9–11 days [16–18]. These studies demonstrate that a deficit of zinc per se must be responsible for the growth retardation. It is well established that a lack of zinc impairs cell division, differentiation and growth in various species, including microorganisms, plants and vertebrates (see [19] for review).
Zn concentrations in plasma and femur and AP activity in plasma, known to be sensitive indicators of Zn status under experimental conditions, responded markedly to the difference in dietary Zn nutrition in both experiments (Tables 3, 6). In Experiment 1, the rats fed the SF diets had the lowest plasma Zn concentrations at either dietary Zn level. This finding may suggest a decreased intestinal Zn availability as a possible explanation for the lower final live weights of the rats consuming the low-Zn SF diet. In Experiment 2, mean plasma Zn concentration was again lower (12%) in the low-Zn SF than in the low-Zn BT group, but the difference failed to be statistically significant. In addition, femur Zn concentrations and plasma AP activities, which reflect Zn status over a longer period than plasma zinc, were comparable between the low-Zn BT and SF groups in either experiment, and thus argue against the idea that enriching the diets with sunflower oil may have lowered Zn availability and hence growth of the animals.
Regarding the effect of dietary Zn depletion on plasma or serum lipids, the literature data are not uniform. Some authors reported that Zn-deficient rats had decreased triglyceride (TAG) concentrations compared to pair-fed or ad libitum-fed control animals [20, 21], whereas others found that Zn depletion did not significantly alter TAG levels [22–24]. In a series of studies, in which young rats were force-fed Zn-deficient diets containing different fat levels and fat sources, TAG concentrations were increased or remained unaffected relative to values of control rats force-fed equal quantities of the same diets with adequate Zn supply [18, 24, 25]. In our experiments, the difference in dietary Zn supply did not significantly affect plasma TAG concentrations regardless of fat content and fat source (Tables 4, 7). Thus, it does not appear that a moderate Zn deficit plays a primary role in modulating TAG levels in circulating blood. In contrast, fat source significantly influenced plasma TAG concentrations. Proposed mechanisms explaining the lower plasma TAG levels in response to the ingestion of polyunsaturated relative to saturated fats include a decreased rate of lipoprotein secretion [26–29] and an increased lipoprotein clearance from circulation [30, 31]. Gene expression studies provide support for both of these mechanisms (see [32] for a recent review).
The effect of Zn deficiency on plasma or serum cholesterol concentrations has also been found to differ among previous studies. In several investigations, Zn depletion of young rats was associated with lower cholesterol levels [20, 21, 33–35], whereas in others cholesterol values of Zn-depleted rats did not differ from those of pair-fed or ad libitum-fed control animals [22, 23, 36–38]. Young rats force-fed Zn-deficient diets in quantities above those voluntarily consumed by Zn-deprived animals showed rather regularly elevated cholesterol levels compared to animals force-fed equivalent amounts of Zn-adequate diets [19, 25]. In Experiment 1 of our study, plasma total and HDL cholesterol concentrations were also higher in the rats consuming the low-Zn, low-fat diet as compared to the corresponding high-Zn CT diet. In both experiments, however, the rats fed the high-fat diets displayed comparable cholesterol levels regardless of dietary Zn level, fat source and energy intake. This finding agrees with a former study in which serum cholesterol concentrations were comparable among rats fed diets containing 20% fat as beef tallow or sunflower oil [39]. A possible explanation for the lower HDL-cholesterol levels in plasma of the animals given the high-fat diets may be the former observation that activation of the peroxisome proliferator-activated receptor alpha (PPARα) decreases circulating HDL in rodents [40].
A few former investigations found that dietary Zn depletion did not alter TAG concentrations in the liver of young rats [33, 41], whereas others reported reduced contents compared to animals fed Zn-adequate diets either ad libitum [21, 38] or in restricted amounts [42]. Our experiments indicate that dietary fat content and level of energy intake have a greater impact on liver lipids than a moderate Zn deficit. In Experiment 1, the livers of the animals fed the high-Zn BT and SF diets had significantly higher TAG contents than those given the corresponding low-Zn diets (Table 4), but their energy intake was also significantly higher due to the ad libitum feeding protocol (Table 3). In Experiment 2, in which intake of the high-Zn diets was restricted to the intake of the respective low-Zn diet, the difference in dietary Zn intake did not alter hepatic TAG concentrations. But the rats offered the high-Zn SF diet for ad libitum intake had a markedly higher hepatic TAG content than those consuming the SF diet in lower amounts (about 27%). Data documented by Cunnane [41] also suggest that a difference in food intake of about 20% may be sufficient to affect TAG levels in the liver of growing rats fed diets supplemented with greatly different levels of zinc.
The fat source was a significant factor for hepatic lipid concentrations in both experiments of our study. The SF diet effected substantially higher TAG concentrations in the liver than the BT diet. This agrees with numerous former investigations reporting that diets containing oils rich in unsaturated fatty acids (including sunflower oil among other vegetable oils) increase hepatic TAG concentrations compared to saturated fats [21, 29, 43, 44] or to diets low in fat [45]. Various mechanisms have been proposed for the differences in hepatic TAG concentration. Monsma and Ney [45] reported that the intestinal absorption of the stearate-rich diets was reduced. A reduced fat digestibility was also evident in our study for the BT diet compared to the other diets (as discussed before). The lower digestibility of the beef tallow, however, must be considered insufficient to explain the lower hepatic TAG levels in our experiments for the following reasons. First, TAG levels of the rats fed the BT diets were comparable to those observed for the rats given the low-fat control diets. Secondly, ME intake per unit weight gain was closely comparable among diets within the same dietary Zn level in both experiments. Finally, other workers demonstrated that PUFA-rich diets reduce the assembly and secretion of VLDL in the liver (see [32, 46] for reviews).
Previous studies, in which low-fat diets were fed to growing rats, showed that dietary Zn depletion of rats does not significantly alter hepatic cholesterol concentrations [21, 33, 47]. The livers of the rats fed the low-fat diets in Experiment 1 of our study also had comparable cholesterol concentrations regardless of the dietary Zn level. This was also true for the high-fat diets in Experiment 2, in which intake of the high-Zn diets was restricted. Therefore, the significant difference in liver cholesterol concentrations between the low-Zn and high-Zn BT and SF groups in Experiment 1 must be attributed to the difference in energy or fat intake. Higher cholesterol concentrations in the liver of young rats in response to an increased dietary fat supply have been observed previously [48, 49]. The rats consuming the SF diets had the highest cholesterol concentrations in both experiments of our study. This effect is consistent with numerous former studies showing higher hepatic cholesterol concentrations in rats fed diets enriched with (15% or more) unsaturated vegetable oils as compared to diets supplemented with saturated fats [44, 48–50]. Increased hepatic cholesterol levels in response to the feeding of diets rich in n-6 PUFA may result from a lower formation and secretion of VLDL as discussed before in the case of hepatic TAG concentrations.
Previous studies with young rats have found that phospholipid (PL) concentrations are barely affected by feeding Zn-deficient diets [18, 41, 47] or by different fat sources [45]. In agreement, the hepatic PL concentrations remained closely comparable in the present study despite substantial differences in zinc, fat and energy intake and dietary fat source. The fatty acid composition of the hepatic PL also reflects a remarkable resistance to the dietary variables, most notable in the similar total percentages of saturated (SFA) across diets. There is no evidence from our data that the conversion of linoleic and α-linolenic acid into longer-chain PUFA might have been impaired by the moderate Zn deficiency, even though linoleic, γ-linolenic (18:3n-6) and docosapentaenoic (20:5n-3) acid were present in significantly higher proportions in the PL of the rats fed the low-Zn diets than in those given the corresponding high-Zn diets. The percentages of arachidonic (20:4n-6) and docosahexaenoic (22:6n-3) acid, the main end products of linoleic and α-linolenic acid, respectively, were closely comparable between the corresponding low- and high-Zn groups. This supports those former studies showing that dietary Zn deficiency per se does not impair the chain elongation/desaturation pathway of essential fatty acids (see [51] for review).
As expected, the diet enriched with sunflower oil, containing linoleic acid as dominant fatty acid, markedly promoted the incorporation of n-6 PUFA, especially arachidonic acid, into the PL, mostly at the expense of docosahexaenoic acid. A significant effect of the dietary fat level is evident among the SFA. The hepatic PL of the rats fed the low-fat diets contained higher proportions of palmitic than stearic acid. The proportions of these two SFA were reversed in the case of the animals consuming the high-fat diets. Two major reasons may explain this shift. First, hepatic de novo lipogenesis was evidently the major source of palmitic acid in the liver of the CT-fed rats, whereas SFA intake was low. In support, the activity of glucose-6-phosphate dehydrogenase, which belongs to the lipogenic enzyme family and closely correlates with the rate of fatty acid synthesis in the liver [15], was very much higher in the CT groups than in the high-fat groups (Table 3). Secondly, stearic acid was presumably desaturated to oleic acid in the liver of the CT-fed animals to a major extent, since its intake was also low, yet its proportions in the PL fraction approximately as high as those in the high-fat groups, for which oleic acid was available in appreciable amounts from dietary intake.
Conclusion
Taken together, the current study shows that diets highly enriched with either beef tallow or sunflower oil have a quantitatively greater impact on plasma and liver lipid composition than a moderate Zn deficiency. Yet, none of the lipid parameters seems to offer a possible explanation why the marginal Zn supply caused a greater growth retardation when the diet contained sunflower oil instead of beef tallow. Fatty acids certainly were the major energy source in either of these groups. However, in the SF diet over 85% of the fatty acids were unsaturated, mainly linoleic acid, whereas the BT diet contained less unsaturated fatty acids (about 52%). Considering the limited dietary supply of carbohydrates and SFA from the SF diet and the need of SFA in the synthesis of membrane and storage lipids, it is conceivable that the SF-fed rats may have largely relied on PUFA as energy source. Mitochondrial and peroxisomal β-oxidation of unsaturated fatty acids, including linoleic and oleic acid, requires Δ3,Δ2 enoyl-CoA isomerase (ECI, EC 5.3.3.8) as key auxiliary enzyme [52, 53]. In the liver of young rats force-fed Zn-deficient diets, mRNA profiling showed that the transcription of genes coding for enzymes of the β-oxidation scheme, including ECI, was markedly reduced compared to Zn-adequate control animals [54, 55]. Further research is needed to investigate whether and to what extent a moderate Zn deficiency may lead to a reduced ECI activity as a possible explanation of the consistently attenuating effect of diets enriched with sunflower oil on weight gain of weanling rats in the present experiments and in a previous study [7].
Abbreviations
- ANOVA:
-
Analysis of variance
- AP:
-
Alkaline phosphatase
- BT:
-
Beef tallow
- CT:
-
Control
- DNL:
-
De novo lipogenesis
- GE:
-
Gross energy
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- HDL-C:
-
High density lipoprotein cholesterol
- ME:
-
Metabolizable energy
- MUFA:
-
Monounsaturated fatty acid(s)
- PUFA:
-
Polyunsaturated fatty acid(s)
- PL:
-
Phospholipids
- SF:
-
Sunflower (seed) oil
- SFA:
-
Saturated fatty acid(s)
- TAG:
-
Triacylglycerol(s)
- TC:
-
Total cholesterol
- VLDL:
-
Very low density lipoproteins
- Zn:
-
Zinc
References
Chesters JK, Quarterman J (1970) Effects of zinc deficiency on food intake and feeding pattern of rats. Br J Nutr 24:1061–1069
Pallauf J, Kirchgessner M (1971) Experimenteller Zinkmangel bei wachsenden Ratten. Z Tierphysiol Tierernährg u Futtermittelkde 28:128–139
Kramer TR, Priske-Anderson M, Johnson SB, Holman AT (1984) Influence of reduced food intake on polyunsaturated fatty acid metabolism in zinc-deficient rats. J Nutr 114:1224–1230
Bettger WJ, Reeves PG, Moscatelli EA, Reynolds G, O’Dell BL (1979) Interaction of zinc and essential fatty acids in the rat. J Nutr 109:480–488
Bettger WJ, Reeves PG, Moscatelli EA, Savage JE, O’Dell BL (1980) Interaction of zinc and polyunsaturated fatty acids in the chick. J Nutr 110:50–58
Huang YS, Cunnane SC, Horrobin DF, Davignon J (1982) Most biological effects of zinc deficiency corrected by γ-linolenic acid (18:3ω6) but not by linoleic acid (C18:2ω6). Atherosclerosis 41:193–207
Weigand E (2011) Fat source affects growth of weanling rats fed high-fat diets low in zinc. J Anim Physiol Anim Nutr. doi:10.1111/j.1439-0396.2010.01114.x
Boesch-Saadatmandi C, Most E, Weigand E (2007) Influence of dietary fat supplementation on the iron utilization in growing rats. Ann Nutr Metabol 51:395–401
Naumann C, Bassler R (2006) Methodenbuch. Band III: Die chemische Untersuchung von Futtermitteln, 3. Auflage. VDLUFA-Verlag, Darmstadt, Germany
Blaxter K (1989) Energy metabolism in animals and man. Cambridge University Press, Cambridge, p 34
Deutsche Gesellschaft für Klinische Chemie (1972) Standardisierung von Methoden zur Bestimmung von Enzymaktivitäten in biologischen Flüssigkeiten. Z Klin Chem Klin Biochem 8:658–660
Lowry OH, Roseborough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 183:265–273
Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90:420–426
Kaluzny MA, Duncan LA, Merrit MV, Epps DE (1985) Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res 26:135–140
Salati SM, Amir-Ahmady B (2001) Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr 21:121–140
Flanagan PR (1984) A model to produce pure zinc deficiency in rats and its use to demonstrate that dietary phytate increases the excretion of endogenous zinc. J Nutr 114:493–502
Park JH, Grandjean CJ, Antonson DL, Vanderhoof JA (1986) Effects of isolated zinc deficiency on the composition of skeletal muscle, liver and bone during growth in rats. J Nutr 116:610–617
Eder K, Kirchgessner M (1996) Effects of zinc deficiency on concentrations of lipids in liver and plasma of rats. Trace Elem Electrolytes 13:60–65
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Koo SI, Williams DA (1981) Relationship between the nutritional status of zinc and cholesterol concentration of serum lipoproteins in adult male rats. Am J Clin Nutr 34:2376–2381
Huang YS, Cunnane SC, Horrobin DF, Davignon J (1982) Most biological effects of zinc deficiency corrected by γ-linolenic acid (18:3ω6) but not by linoleic acid (18:2ω6). Atherosclerosis 41:193–207
Roth HP, Kirchgessner M (1977) Zum Einfluss von Zinkmangel auf den Fettstoffwechsel. Int J Vit Nutr Res 47:277–283
Schneeman BO, Lacy D, Ney D, Lefevre ML, Keen CL, Lonnerdal B, Hurley LS (1986) Similar effects of zinc deficiency and restricted feeding on plasma lipids and lipoproteins in rats. J Nutr 116:1889–1895
Eder K, Kirchgessner M (1997) Concentrations of lipids in plasma and lipoproteins and oxidative susceptibility of low-density lipoproteins in zinc-deficient rats fed linseed oil or olive oil. J Nutr Biochem 8:46–468
Kettler SI, Eder K, Kirchgessner M (2000) Zinc deficiency and the activities of lipoprotein lipase in plasma and tissues of rats force-fed diets with coconut oil or fish oil. J Nutr Biochem 11:132–138
Engelberg H (1966) Mechanisms involved in the reduction of serum triglycerides in man upon adding unsaturated fats to the normal diet. Metabolism 15:796–807
Chait A, Onitiri A, Nicoll A, Rabaya E, Davies J, Lewis B (1974) Reduction of serum triglyceride levels by polyunsaturated fat. Studies on the mode of action and on very low density lipoprotein composition. Atherosclerosis 20:347–364
Shimomura Y, Tamura T, Suzuki M (1990) Less body fat accumulation in rats fed a safflower oil diet than in rats fed a beef tallow diet. J Nutr 120:1291–1296
Lai HC, Lasekan JB, Yang H, Clayton MK, Ney DM (1991) In vivo determination of triglyceride secretion using radioactive glycerol in rats fed different dietary saturated fats. Lipids 26:824–830
Green MH, Massaro ER, Green JB (1984) Multicompartmental analysis of the effects of dietary fat saturation and cholesterol on absorptive lipoprotein metabolism in the rat. Am J Clin Nutr 40:82–94
Groat PHE, de Boer BCJ, Haddeman E, Houtsmuller UMT, Hülsmann WC (1988) Effect of dietary fat composition on the metabolism of triacylglycerol-rich plasma lipoproteins in the postprandial phase in meal-fed rats. J Lipid Res 29:541–551
Rakhshandehroo M, Knoch B, Müller M, Kersten S (2010) Peroxisome proliferator-activated receptor alpha target genes. PPAR Res 2010:1–20. Article ID 612089. doi:10.1155/2010/612089
Patel PB, Chung RA, Lu JY (1975) Effect of zinc deficiency on serum and liver cholesterol in the female rat. Nutr Rep Int 12:205–210
Frimpong NA, Magee AC (1981) Effects of dietary copper and zinc on serum lipid parameters of young male rats. Nutr Rep Int 35:551–559
Koo SI, Lee CC (1988) Compositional changes in plasma high-density lipoprotein particles in marginally zinc-deficient male rats. Am J Clin Nutr 47:909–919
Quarterman J, Florence E (1972) Observations on glucose tolerance and plasma levels of free fatty acids and insulin in the zinc-deficient rat. Br J Nutr 28:75–79
Koo SI, Henderson DA, Algilani K, Norvell JE (1985) Effect of marginal zinc deficiency on the morphological characteristics of intestinal nascent chylomicrons and distribution of soluble apoproteins of chylomicrons. Am J Clin Nutr 42:671–680
Lefevre M, Keen CL, Lönnerdal B, Hurley LS, Schneeman BO (1985) different effects of zinc and copper deficiency on composition of plasma high density lipoproteins in rats. J Nutr 115:359–368
Portillo MP, Chavarri M, Duran D, Rodriguez VM, Macarulla MT (2001) Differential effects of diets that provide different lipid sources on hepatic lipogenic activities in rats under ad libitum or restricted feeding. Nutrition 17:467–473
Vu-Dac N, Chopin-Delannoy S, Gervois P et al (1998) The nuclear receptor peroxisome proliferator-activated receptors α and Rev-erbα mediate the species specific regulation of apolipoprotein A-I expression by fibrates. J Biol Chem 273:25713–25720
Cunnane SC (1988) Evidence that adverse effects of zinc deficiency on essential fatty acid composition in rats are independent of food intake. Br J Nutr 59:273–278
Fogerty AC, Ford GL, Dreosti IE, Tinsley IJ (1985) Zinc deficiency and fatty acid composition of tissue lipids. Nutr Rep Int 32:1009–1019
Monsma CC, Ney DM (1993) Interrelationship of stearic acid content and triacylglycerol composition of lard, beef tallow and cocoa butter in rats. Lipids 28:539–547
Monsma CC, Gallaher DD, Ney DM (1996) Reduced digestibility of beef tallow and cocoa butter affects bile acid excretion and reduces hepatic esterified cholesterol in rats. J Nutr 126:2028–2035
Rustan AC, Christiansen EN, Drevon CA (1992) Serum lipids, hepatic glycerolipid metabolism and peroxisomal fatty acid oxidation in rats fed omega-3 and omega-6 fatty acids. Biochem J 283:333–339
Kersten S (2008) Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res 2008. Article ID 132960. doi:10.1155/2008/132960
Clejan S, Maddaiah VT, Castro-Magana M, Collipp PJ (1981) Zinc deficiency induced changes in the composition of microsomal membranes and in the enzymatic regulation of glycerolipid synthesis. Lipids 16:454–460
Reiser R, Williams MC, Sorrels MF, Murty NL (1963) Biosynthesis of fatty acids and cholesterol as related to diet fat. Arch Biochem Biophys 102:276–285
Wiggers KD, Richard MJ, Stewart JW, Jacobson NL, Berger PJ (1977) Type and amount of dietary fat affect relative concentration of cholesterol in blood and other tissues of rats. Atherosclerosis 27:27–34
Kellog TF (1974) Steroid balance and tissue cholesterol accumulation in germfree and conventional rats fed diets containing saturated and polyunsaturated fats. J Lipid Res 15:574–579
Eder K, Kirchgessner M (1996) Dietary zinc deficiency and fatty acid metabolism in rats. Nutr Res 16:1179–1189
Gurvitz A, Wabnegger L, Yagi AI, Binder M, Hartig A, Ruis H, Hamilton B, Dawes IW, Hiltunen JK, Rottensteiner H (1999) Function of human mitochondrial 2,4-dienoyl-CoA reductase and rat monofunctional Δ3-Δ2-enoyl-CoA isomerase in β-oxidation of unsaturated fatty acids. Biochem J 344:903–914
Janssen U, Stoffel W (2002) Disruption of mitochondrial β-oxidation of unsaturated fatty acids in the 3, 2-trans-enoyl-CoA isomerase-deficient mouse. J Biol Chem 277:19579–19584
Tom Dieck H, Döring F, Roth HP, Daniel H (2003) Changes in rat hepatic gene expression in response to zinc deficiency as assessed by DNA arrays. J Nutr 133:1004–1010
Tom Dieck H, Döring F, Fuchs D, Roth HP, Daniel H (2005) Transcriptome and proteome analysis identifies the pathways that increase hepatic lipid accumulation in zinc-deficient rats. J Nutr 135:199–205
Acknowledgments
We are grateful for the technical support provided by Dr. Erika Most.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Weigand, E., Boesch-Saadatmandi, C. Interaction Between Marginal Zinc and High Fat Supply on Lipid Metabolism and Growth of Weanling Rats. Lipids 47, 291–302 (2012). https://doi.org/10.1007/s11745-011-3629-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3629-y