Abstract
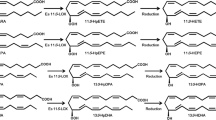
A very long chain polyunsaturated hydrocarbon, hentriacontanonaene (C31:9), was detected in an eicosapentaenoic acid (EPA)-producing marine bacterium, which was isolated from the mid-latitude seashore of Hokkaido, Japan, and was tentatively identified as mesophilic Shewanella sp. strain osh08 from 16S rRNA gene sequencing. The geometry and position of the double bonds in this compound were determined physicochemically to be all cis at positions 3, 6, 9, 12, 15, 19, 22, 25, and 28. Although C31:9 was detected in all of the seven EPA- or/and docosahexaenoic acid-producing bacteria tested, an EPA-deficient mutant (strain IK-1Δ8) of one of these bacteria had no C31:9. Strain IK-1Δ8 had defects in the pfaD gene, one of the five pfa genes responsible for the biosynthesis of EPA. Although Escherichia coli DH5α does not produce EPA or DHA inherently, cells transformed with the pfa genes responsible for the biosynthesis of EPA and DHA produced EPA and DHA, respectively, but not C31:9. These results suggest that the Pfa protein complex is involved in the biosynthesis of C31:9 and that pfa genes must not be the only genes responsible for the formation of C31:9. In this report, we determined for the first time the molecular structure of the C31:9 and discuss the possible biosynthetic pathways of this compound.







Similar content being viewed by others
Abbreviations
- CI:
-
Chemical ionization
- CI-GC/MS:
-
Chemical ionization–mass spectrometry
- DHA:
-
Docosahexaenoic acid
- EI:
-
Electron impact ionization
- EI-GC/MS:
-
Electron impact ionization–mass spectrometry
- EPA:
-
Eicosapentaenoic acid
- FAME:
-
Fatty acid methyl ester
- GC/MS:
-
Gas chromatography–mass spectrometry
- GLC:
-
Gas–liquid chromatography
- HPLC:
-
High-performance liquid chromatography
- PUHC:
-
Polyunsaturated hydrocarbon
- PUFA:
-
Polyunsaturated fatty acid
References
Nichols DS, Nichols PD, McMeekin TA (1995) A new n-C31:9 polyene hydrocarbon from Antarctic bacteria. FEMS Microbiol Lett 125:281–285
Tamaoka J, Yanagibayashi M, Kato C, Horikoshi K (1998) A polyunsaturated hydrocarbon hentriacontanonaene (C31H46) from a deep-sea bacterium strain DSS12. In: Atlantic Canada Society for Microbial Ecology and International Committee on Microbial Ecology (eds) Program & Abstracts. Eighth international symposium on microbial ecology, Halifax, p 319
Okuyama H, Orikasa Y, Nishida T, Morita N (2007) Bacterial genes responsible for the biosynthesis of eicosapentaenoic and docosahexaenoic acids and their heterologous expression. Appl Environ Microbiol 73:665–670
Blumer M, Mullin MM, Guillard RRC (1970) A polyunsaturated hydrocarbon (3, 6, 9, 12, 15, 18-heneicosahexaene) in the marine food web. Marine Biol 6:226–235
Lee RF, Loeblich AR III (1971) Distribution of 21:6 hydrocarbon and its relationship to 22:6 fatty acid in algae. Phytochemistry 10:593–602
Kolattukudy PE, Croteau R, Brown L (1974) Structure and biosynthesis of cuticular lipids. Plant Physiol 53:670–677
ZoBell CE (1946) Marine microbiology, a monograph on hydrobacteriology. Chronica Botanica Co., Waltham
Yumoto I, Kawasaki K, Iwata H, Matsuyama H, Okuyama H (1998) Assignment of Vibrio sp. strain ABE-1 to Colwellia maris sp. nov., a new psychrophilic bacterium. Int J Syst Bacteriol 48:1357–1362
Satomi M, Oikawa H, Yano Y (2003) Shewanella marinintestina sp. nov., Shewanella schlegeliana sp. nov. and Shewanella sairae sp. nov., novel eicosapentaenoic-acid-producing marine bacteria isolated from sea-animal intestines. Int J Syst Evol Microbiol 53:491–499
Hirota K, Nodasaka Y, Orikasa Y, Okuyama H, Yumoto I (2005) Shewanella pneumatophori sp. nov., eicosapentanoic-acid-producing marine bacterium isolated from pacific mackerel (Pneumatophorus japonicus) intestine. Int J Syst Evol Microbiol 55:2355–2359
Yumoto I, Yamazaki K, Hishinuma M, Nodasaka Y, Suemori A, Nakajima K, Inoue N, Kawasaki K (2001) Pseudomonas alcaliphila sp. nov., a novel facultatively psychrophilic alkaliphile isolated from seawater. Int J Syst Evol Microbiol 51:349–355
Yumoto I, Kusano T, Shingyo T, Nodasaka Y, Matsuyama H, Okuyama H (2001) Assignment of Pseudomonas sp. strain E-3 to Pseudomonas psychrophila sp. nov., a new facultatively psychrophilic bacterium. Extremophiles 5:343–349
Yumoto I, Iwata H, Sawabe T, Ueno K, Ichise N, Matsuyama H, Okuyama H, Kawasaki K (1999) Characterization of a facultatively psychrophilic bacterium, Vibrio rumoiensis sp. nov., that exhibits high catalase activity. Appl Environ Microbiol 65:67–72
Wongsa P, Tanaka M, Ueno A, Hasanuzzaman M, Yumoto I, Okuyama H (2004) Isolation and characterization of novel strains of Pseudomonas aeruginosa and Serratia marcescens possessing high efficiency to degrade gasoline, kerosene, diesel oil, and lubricating oil. Curr Microbiol 49:415–422
Perveen Z, Ando H, Ueno A, Ito Y, Yamamoto M, Yamada Y, Takagi T, Kaneko T, Kogame K, Okuyama H (2006) Isolation and characterization of a novel thraustochytrid–like microorganism that efficiently produces docosahexaenoic acid. Biotechnol Lett 28:197–202
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T (1997) Production of high yields of docosahexaenoic acid by Schizochytrium limacinum strain SR21. J Am Oil Chem 74:1431–1434
Chapman D (1965) Infrared spectroscopy of lipids. 15th Annual summer program symposium on quantitative methodology in lipid research. Part II. J Am Oil Chem Soc 42:353–371
Park MO, Tanabe M, Hirata K, Miyamoto K (2001) Isolation and characterization of a bacterium that produces hydrocarbons extracellularly which a equivalent to light oil. Appl Microbiol Biotechnol 56:448–452
Ueda N, Ando S, Hayashi A, Yano I, Hayashi M (1974) Analysis and identification of lipids. In: The Japanese Biochemical Society (ed) Collection of articles on experiments in biochemistry, 1st series, 3. Chemistry of lipids. Tokyo Kagaku Dozin Co., Ltd., Tokyo, pp 75–178 (in Japanese)
Sato D, Ando Y, Tsujimoto R, Kawasaki K (2001) Identification of novel nonmethylene-interrupted fatty acids, 7E, 13E-20:2, 7E, 13E, 17Z-20:3, 9E, 15E, 19Z-22:3, and 4Z, 9E, 15E, 19Z-22:4, in Ophiuroidea (brittle star) lipids. Lipids 36:1371–1375
Okuyama H, Sasaki S, Higashi S, Murata N (1990) A trans-unsaturated fatty acid in a psychrophilic bacterium, Vibrio sp. strain ABE-1. J Bacteriol 172:3515–3518
Oldham NJ, Svatoš A (1999) Determination of the double bond position in functionalized monoenes by chemical ionization ion-trap mass spectrometry using acetonitrile as a reagent gas. Rapid Commun Mass Spectrom 13:331–336
Moneti G, Pieraccini G, Dani F, Catinella S, Traldi P (1996) Acetonitrile as an effective reactant species for positive-ion chemical ionization of hydrocarbons by ion-trap mass spectrometry. Rapid Commun Mass Spectrom 10:167–170
Moneti G, Pieraccini G, Dani F, Turillazzi S, Favretto D, Traldi P (1997) Ion-molecule reactions of ionic species from acetonitrile with unsaturated hydrocarbons for the identification of the double-bond position using an ion trap. J Mass Spectrom 32:1371–1373
Orikasa Y, Nishida T, Hase A, Watanabe K, Morita N, Okuyama H (2006) A phosphopantetheinyl transferase gene responsible for biosynthesis of n-3 polyunsaturated fatty acids from Moritella marina strain MP-1. FEBS Lett 580:4423–4429
Shibahara A, Yamamoto K, Nakayama T, Kajimoto G (1985) Rapid determination of double bond positions in monounsaturated fatty acids by GC–MS and its application of fatty acid analysis. J Jpn Oil Chem Soc 34:618–625
Watanabe K, Ishikawa C, Yazawa K, Kondo K, Kawaguchi A (1996) Fatty acid and lipid composition of an eicosapentaenoic acid-producing marine bacterium. J Mar Biotechnol 4:104–112
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Sambrook J, Russell DW (2001) Molecular Cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Ito H, Hosokawa R, Morikawa M, Okuyama H (2008) A turbine oil-degrading bacterial consortium from soils of oil fields and its characteristics. Int Biodeterior Biodegradation 61:223–232
Wiegel J (1990) Temperature spans for growth: hypothesis and discussion. FEMS Microbiol Lett 75:155–169
Ivanova EP, Sawabe T, Gorshkova NM, Svetashev VI, Mikhailov VV, Hicolau DV, Christen R (2001) Shewanella japonica sp. nov. Int J Syst Evol Microbiol 51:1027–1033
Ivanova EP, Gorshkova NM, Bowman JP, Lysenko AM, Zhukova NV, Sergeev AF, Mikhailov VV, Nicolau DV (2004) Shewanella pacifica sp. nov., a polyunsaturated fatty acid-producing bacterium isolated from sea water. Int J Syst Evol Microbiol 54:1083–1087
Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, MacGregor BJ, Ringelberg DB, White DC, Nishijima M, Sana H, Burghardt J, Stackebrandt E, Nelson KH (1999) Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol 2:705–724
Abboud R, Popa R, Souza-Egipsy V, Giometti CS, Tollaksen S, Mosher JJ, Findlay RH, Nealson KH (2005) Low-temperature growth of Shewanella oneidensis MR-1. Appl Environ Microbiol 71:811–816
Heidelberg J, Paulsen I, Nelson K, Gaidos E, Nelson WC, Read TD, Eisen JA, Seshadri R, Ward N, Methe B, Clayton RA, Meyer T, Tsapin A, Scott J, Beanan M, Brinkac L, Daugherty S, DeBoy RT, Dodson RJ, Durkin AS, Haft DH, Kolonay JF, Madupu R, Peterson JD, Umayam LA, White O, Wolf AM, Vamathevan J, Weidman J, Impraim M, Lee K, Berry K, Lee C, Mueller J, Khouri H, Gill J, Utterback TR, McDonald LA, Feldblyum TV, Smith HO, Venter JC, Nealson KH, Fraser CM (2002) Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotechnol 20:1118–1123
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Nishida T, Morita N, Yano Y, Orikasa Y, Okuyama H (2007) The antioxidative function of eicosapentaenoic acid in a marine bacterium, Shewanella marinintestina IK-1. FEBS Lett 581:4212–4216
Ootaki M, Morita N, Nishida T, Tanaka M, Hase A, Yano Y, Yamada A, Yu R, Watanabe K, Okuyama H (2003) Genes and pathways involved in biosynthesis of eicosapentaenoic and docosahexaenoic acids in bacteria. In: Murata N, Nishida I, Yamada M, Sekiya J, Okuyama H, Wada H (eds) Advanced Researches of Plant Lipids. Kluwer, Tokyo, pp 49–52
Orikasa Y, Tanaka M, Sugihara S, Hori R, Nishida T, Ueno A, Morita N, Yano Y, Yamamoto K, Shibahara A, Hayashi H, Yamada Y, Yamada A, Yu R, Watanabe K, Okuyama H (2009) pfaB Products determine the molecular species produced in bacterial polyunsaturated fatty acid biosynthesis. FEMS Microbiol Lett 295:170–176
Albro PW, Dittmer JC (1969) Biochemistry of long-chain, nonisoprenoid hydrocarbons. III. Metabolic relation of long-chain fatty acids and hydrocarbons and other aspects of hydrocarbon metabolism in Sarcina lutea. Biochemistry 8:1913–1918
Ladygina N, Dedyukhina EG, Vainshtein MB (2006) A review on microbial synthesis of hydrocarbons. Process Biochem 41:1001–1014
Albro PW, Dittmer JC (1969) Biochemistry of long-chain, nonisoprenoid hydrocarbons. IV. Characteristics of synthesis by a cell-free preparation of Sarcina lutea. Biochemistry 8:3317–3324
Orikasa Y (2007) Molecular biological and biochemical studies on microbial genes responsible for the biosynthesis of long chain n-3 polyunsaturated fatty acids. PhD thesis, Hokkaido University, Sapporo, Japan
Acknowledgments
The infrared analysis of C31:9 was carried out using the apparatus of the OPEN FACILITY, Hokkaido University Sousei Hall with extensive assistance by and discussion with Dr. Y. Matsuo. The authors would like to express their appreciation to Drs. K. Shimazu and K. Nakata of the Faculty of Environmental Earth Science, Hokkaido University for their discussions throughout the work. This work was partly supported by a grant from the National Institute of Polar Research and a Grant-in-Aid from Hokkaido Innovation through Nanotechnology Support (HINTS).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sugihara, S., Hori, R., Nakanowatari, H. et al. Possible Biosynthetic Pathways for all cis-3,6,9,12,15,19,22,25,28-Hentriacontanonaene in Bacteria. Lipids 45, 167–177 (2010). https://doi.org/10.1007/s11745-009-3380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-009-3380-9