Abstract
Mislabeling of farmed and wild salmon sold in markets has been reported. Since the fatty acid content of fish may influence human health and thus consumer behavior, a simplified method to identify wild and farmed salmon is necessary. Several studies have demonstrated differences in lipid profiles between farmed and wild salmon but no data exists validating these differences with government-approved methods to accurately identify the origin of these fish. Current methods are both expensive and complicated, using highly specialized equipment not commonly available. Therefore, we developed a testing protocol using gas chromatography (GC), to determine the origin of salmon using fatty acid profiles. We also compared the GC method with the currently approved FDA (United States Food and Drug Administration) technique that uses analysis of carotenoid optical isomers and found 100% agreement. Statistical validation (n = 30) was obtained showing elevated 18:2n-6 (z = 4.56; P = 0.0001) and decreased 20:1n-9 (z = 1.79; P = 0.07) in farmed samples. The method is suitable for wide adaptation because fatty acid methyl ester analysis is a well-established procedure in labs that conduct analysis of lipid composition and food constituents. GC analysis for determining the origin of North American salmon compared favorably with the astaxanthin isomer technique used by the FDA and showed that the fatty acid 18:2n-6 was the key indicator associated with the origin of these salmon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Farmed Atlantic salmon (Salmo salar) production currently provides approximately 50% of world-wide salmon consumption [1]. This popularity is due, in part, to the year-round availability of fresh farmed salmon and the low-cost which can be half that of wild salmon [2]. A direct result of these lower prices is greater availability of a high omega three fish product to the public. Additionally, as the catch of wild salmon becomes more variable, farmed salmon may provide a more stable supply. However, some concern exists over ecological damage from salmon cultivation. Extensive salmon farming may result in negative effects including dwindling fisheries of the small pelagic fish used as feed, interruption of salmon breeding patterns, dilution of the wild salmon gene pool by escaped farmed salmon, and transmission of infections from farmed to wild populations [3–7]. Farmed salmon have been reported to contain on average much higher levels of polychlorinated biphenyls (PCBs) and other nonpolar contaminants than their wild counterparts even after correction for the higher fat content of the farmed salmon [8, 9]. It may be possible to solve these problems; some farmed salmon, such as those from Chile, have very favorable contamination profiles which may rival wild salmon such as Chinook [10–12]. Mozaffarian and Rimm make the valid point that some common foods also may have similar concentrations of PCBs to farmed salmon and although eating such fish may have certain risks, they believe the health benefits exceed the dangers [2].
Contaminated fishmeal and depletion of pelagic fish used in fishmeal may be motivating commercial fish farmers to develop alternate feeding strategies. The nutritional requirements of farmed salmon can be met in part by the substitution of plant derived oils and protein such as rapeseed, corn, palm, or soybean [13–16]. Some studies indicate that resultant feeds containing up to 100% terrestrial plant oils are well tolerated, support normal growth rates, and provide filets with acceptable flavor [14, 15, 17].
Deep cold water fish such as salmon have low delta-6 desaturase enzyme activity and therefore have a limited ability to convert linoleic acid (18:2n-6, LNA) to arachidonic acid (20:4n-6, ARA), and linolenic acid (18:3n-3, ALA) to eicosapentaenoic acid (20:5n-3, EPA) and docosahexaenoic acid (22:6n-3, DHA) [15, 17, 18]. Consequently, farmed deep cold water fish must be fed a “finishing diet” containing increased amounts of fish oils for the last few weeks before slaughter. This diet maintains the most beneficial fatty acid balance, including the higher levels of EPA and DHA that are fundamental to the health benefits of fatty fish consumption [13, 14, 19].
To maintain the characteristic reddish-orange color of their flesh, salmon must consume the carotenoids astaxanthin and canthaxanthin. Farmed salmon are fed fishmeal supplemented with various isomers of these carotenoids; wild salmon assimilate carotenoids by consuming krill. It is interesting to note that the color differences between wild and farmed salmon are often indistinguishable by visual inspection and thus advanced analytical techniques are required for authentication [14, 19–23].
Interest in farmed and wild salmon by both consumers and scientists has led to increased pressure to determine accurately the origin of different fish provided to the marketplace. In 1998, the United States Food and Drug Administration (FDA) developed an accurate method to authenticate the origin of salmon by measuring unique isomer ratios of astaxanthin [20]. The origin of the salmon in our study was verified by the FDA method that uses high performance liquid chromatography (HPLC) analysis of astaxanthin isomers. The HPLC method requires specialized equipment such as specific chiral columns or derivatization with chiral reagents. Our objective was to establish a facile gas chromatography (GC) fatty acid analysis technique for distinguishing wild and farmed salmon that could be readily implemented with the resources of a typical biochemistry laboratory.
Materials and Methods
Thirty salmon filet samples were provided to our laboratory by Craft Technologies in Wilson, NC, USA. These samples had been collected during the interval between 2004 and 2006 at various fish markets in the Northeastern and central United States. All samples were stored frozen at −80 °C and transported on dry ice.
Craft Technologies tested all samples for carotenoid profiles using the FDA method which includes a combination of normal-phase and chiral HPLC as described in references [20, 24]. Astaxanthin was extracted from the fish samples by homogenization with acetone. After centrifugation to remove protein debris, the extract was injected on a Chromegabond diol column (ES Industries), 15 × 0.46 cm, 5 μm particle size, with 96% hexane/4% isopropanol mobile phase, at 1.5 mL/min flow rate. Detection was at 450 nm. The astaxanthin fraction was collected, the solvent was evaporated, and the sample re-dissolved in 85% hexane/15% acetone for the next HPLC step. The isolated astaxanthin fraction was re-analyzed for enantiomer composition with two chiral columns in series: Chiralcel C18 250 × 4.6 mm (Diacel Chemical Industries, Ltd), 5 μm particle size. The mobile phase was 85% hexane/15% acetone, at 0.8 mL/min flow rate, and detection was at 450 nm [20].
Preparation of Fatty Acid Methyl Esters
One gram of each salmon filet was minced with single-edged razor blades and homogenized in 10 mL of 0.15 M NaCl, using a handheld glass Potter–Eljvehem homogenizer. For extraction of lipids, 1 mL of the tissue homogenate was vortexed with 2 mL of chloroform/methanol (2:1) and 0.1% BHT as antioxidant. After centrifugation, the lower organic layer was collected and evaporated in an 8-mL vial under nitrogen.
The methylation reagent was generated by mixing 1 mL of acetyl chloride with 30 mL of MeOH, and was used within 5 days of preparation. One milliliter of this reagent was added to the residue from extraction, along with 200 μL of hexane in a tightly sealed 10-mL Teflon-capped glass vial, and the sample was heated at 100 °C for 1 h to convert fatty acids to methyl esters. After addition of 1 mL of 0.15 M sodium bicarbonate and 2 mL of hexane, the sample was vortexed, centrifuged, and the upper hexane layer was transferred to a second 10-mL glass vial, evaporated under a nitrogen stream, and dissolved in 200 μL chloroform for GC analysis.
GC Analysis and Peak Identification
Samples were analyzed on an HP 5890 GC (Avondale, PA, USA), equipped with a flame-ionization detector. The column was a DB-23, 30M × 0.25 mm i.d. with film thickness of 0.25 μM (J & W Scientific, Folsom, CA, USA). The stationary phase was a (50% phenyl)-methylpolysiloxane. Helium was used as the carrier gas at 30 psi column pressure. One microlitre of sample was injected using an HP-6331 auto-injector, with a 1:15 split ratio. Initial column temperature was 160 °C, with a 1 °C/min gradient to 200 °C, and an additional 5 min at 200 °C. The injector and detector were set at 240 °C.
Calibration was done with fatty acid methyl ester standards from Nu-Chek Prep (Elysian, MN, USA). Chromatograms were collected and integrated with Lab-Calc software (Galactic Industries, Salem, NH, USA), on an IBM-PC. The total peak areas of the following fatty acids were determined: 14:0 myristic acid, 16:0 palmitic acid, 18:0 stearic acid, 18:1n-9 oleic acid, 18:1n-7 vaccenic acid, 18:2n-6 linoleic acid, 20:1n-9 eicosenoic acid, 20:4n-6 arachidonic acid, 20:5n-3 eicosapentaenoic acid, and 22:6n-3 docosahexaenoic acid. Each peak of interest was reported as weight percent of the sum of these major fatty acid components.
Research Design and Selection of Criterion Value
Our research design required two sample groups. The initial batch had five wild and five farmed salmon samples with known identities as determined by carotenoid chiral isomer ratios. This first batch was used to establish a criterion value for categorizing salmon as either wild or farmed. The second batch contained 20 samples whose categorization (wild or farmed) was not disclosed to the team analyzing the fatty acid composition.
After reviewing several fatty acids that might be used for discrimination within the sample of ten salmon with known classifications, we determined that LNA was the best indicator, as there was no overlap of LNA concentrations within the distribution of the pre-identified wild and farmed salmon. We then calculated the criterion value by choosing the lowest LNA (9.96%) value for the known farmed samples and the highest value (2.19%) from the known wild samples, and then taking the midpoint between those two values (6.1%).
We then tested the criterion value of 6.1% LNA on the remaining 20 unknown samples. The “unknown” samples were sent by Craft Technologies without any indication as to their carotenoid ratios or their classification as wild or farmed. Craft Technologies had previously categorized these 20 samples using the FDA-approved carotenoid chiral isomer ratios test.
Using the proposed 6.1% LNA criterion value, the 20 unknown samples were classified as farmed (LNA over 6.1%) or wild (LNA below 6.1%). The classifications for each of these 20 samples were then unblinded by Craft Technologies. The match between the criterion value classification and the carotenoid chiral isomer ratios classification was 100%.
Fatty Acid Statistics
Significant differences between farmed and wild salmon sample fatty acid ratios were noted by comparison of chromatographic peak areas. These results were recorded as percent of total weight of fatty acids. Difference of proportions tests were calculated on each fatty acid to determine statistical significance between farmed and wild salmon samples. This test produces z scores which are subsequently converted to P values, see Table 1 [25, 26]. These calculations were carried out using Microsoft Excel version 2000.
Results
The fatty acid results for the initial sample of ten salmon that were analyzed are shown in Table 2. These salmon had all been pre-identified by chiral carotenoid analysis.
The highest LNA for wild salmon in this sample was 2.19%, and the lowest LNA for farmed salmon was 9.96%.
Representative carotenoid analyses are shown in Fig. 1. Wild salmon ingest predominantly a mixture of a astaxanthin from copepods and krill, which contain mostly the 3R,3′R and 3S,3′S astaxanthin isomers, and very little of the 3R,3′S isomer. Salmon fed yeast astaxanthin in their diet show primarily the 3S,3′S isomer, and salmon fed synthetic astaxanthin show an abundance of the central “meso” peak on the HPLC analysis, the 3R,3′S isomer.
Table 1 summarizes the fatty acid retention times and percent of total for each of the fatty acids reported in this analysis. This table was assembled after all the salmon samples had been classified as wild or farmed, using the criterion value of 6.1% LNA obtained from the initial set of identified salmon. There was complete agreement between assignment using LNA content and assignment using carotenoid analysis. These results are provided graphically in the histogram in Fig. 2, which emphasizes that LNA was the major peak that differs between wild and farmed.
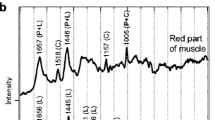
Representative fatty acid profiles by GC of wild and farmed salmon are shown in Fig. 3. The elevated 18:2n-6 peak is highly distinctive and easily identifiable on the trace of the cultivated sample. Farmed samples may have lower 20:1n-9, but this fatty acid was not statistically significant (P = 0.07). The other fatty acid components are generally comparable between wild and cultivated salmon and are not statistically different. Also, there was a non-significant trend toward decreasing percent of several major fatty acids in cultivated salmon reflecting the presence of increased 18:2n-6 (see Table 1; Fig. 2). The 22:1n-11 peak (sometimes called cetoleic acid), which elutes at 26.8 min in Fig. 3, is more abundant in many of the wild samples than in the farmed samples, but exhibits a high variance between samples. The peak is present at low abundance in all the farmed samples (1–2%) and in some wild samples. This may reflect the fact that some of the wild-caught fish consumed copepods or other foods rich in 22:1n-11. Although of potential interest, this fatty acid cannot be reliably used for classifying the origin of the fish. Thomas et al. [27] also observed a much greater variance for 22:1n-11 in wild salmon than the other fatty acids reported in their study.
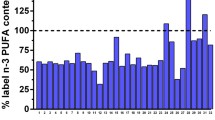
The percentage of LNA was plotted separately for all samples in Fig. 4; one sample, classified as wild by the LNA criterion, had a somewhat higher LNA content (4.3%) than the other 14 wild samples. This sample had an unusual carotenoid profile as well, intermediate between what is typically seen for wild and farmed salmon. Based on both the LNA content and carotenoid profile, we hypothesize that this sample is a farmed salmon that escaped and consumed a natural diet in the wild for an extended period.
Discussion
Wild and farmed salmon are similar in appearance and therefore it may be difficult for consumers to distinguish between them by visual inspection. For this reason, the FDA developed the accurate, but complicated, HPLC method using chiral isomers of astaxanthin to identify farmed salmon. In our study, we analyzed fatty acid profiles in salmon samples that had been pre-identified using the FDA HPLC method.
Trends in the feeding practices for cultivated salmon could decrease the reliability of carotenoid analysis for determination of fish origins since wild krill and Haematococcus microalgae are being employed as part of the fish rations, and the astaxanthin isomer ratio in these fish could resemble that of wild salmon (see Fig. 1 for typical astaxanthin profiles) [23, 28]. By contrast, fatty acid analysis should continue to be a reliable method for classification. For example, Bell et al. [16] reported that when salmon flesh fatty acid content is plotted against their dietary intake of fatty acid for 20:5n-3 and 22:1, r = 1.0, while other fatty acids such as 18:2n-6 and 18:1n-9 result in the slightly lower flesh values of r = 0.97 and 0.98, respectively. Current trends in fish feed development may lead to increased levels of 18:2n-6 in farmed fish, emphasizing the utility of fatty acid analysis in identifying farmed and wild salmon [15, 16, 19, 22, 29, 30].
Due primarily to price pressures, mislabeling of the origins of commercially sold salmon has been previously reported [27, 31]. Without quick, accurate, inexpensive, and commonly available testing procedures to authenticate wild and farmed salmon, mislabeling could become increasingly problematic. It therefore may be useful that readily available procedures such as fatty acid analysis by GC be applied to this question, and it should be possible to accomplish this analysis using the facilities in many nutrition and food science laboratories.
Our identification of farmed and wild salmon samples using fatty acid analysis demonstrated 100% agreement with the carotenoid isomer identification technique established by the FDA. The higher percent of 18:2n-6 in the farmed salmon flesh is an excellent marker for distinguishing farmed and wild salmon, based on current feeding practices. Statistically, the percent of 18:2n-6 had a value of P = 0.0001 when the differences between all farmed and all wild (n = 30) samples were compared, indicating a high level of certainty that the amount of this fatty acid is dissimilar between the two groups of salmon. The only other fatty acid that approached statistical significance was the 20:1n-9, but it failed to reach 95% significance (P = 0.07). We chose not employ it as a marker for identification.
As noted earlier, the fatty acids that salmon consume are assimilated into their flesh in proportion to dietary content (less any amount used for energy derived through beta-oxidation) [19]. Cultivated fish are currently being fed meal formulations with increasing ratios of plant-based oils [15, 16, 19, 22]. This change may reduce persistent organic contaminants, decrease the cost of fish feed, and ultimately increase the sustainability of aquaculture as a whole by preserving threatened pelagic fisheries [14, 16]. The practice of switching from fish oils to terrestrial plant oils leads directly to the increased 18:2n-6 content reported here, since 18:2n-6 is a major constituent of corn, rapeseed, and soybean oils that are typically used as components of the diet of farmed salmon. North American suppliers of salmon feed may use largely soybean oil and corn oil, consistent with the elevated n-6 fatty acid content of salmon feed in North America [32]. Salmon feed employed in Europe may have a different fatty acid profile, and the 18:2n-6 fatty acid content of farmed salmon from Scotland can be lower for some fish than farmed salmon from North American [8, 32].
Several studies that address the content of highly unsaturated fatty acids such as EPA and DHA have found that the relative percentages of these fatty acids were similar between farmed and wild salmon, but the total content of these fatty acids was higher in farmed fish because of the higher total lipid content [8, 22, 30, 33–35]. The percentage of EPA and DHA was not statistically different between wild and farmed salmon in the samples reported here, but we did demonstrate a consistently higher percentage of 18:2n-6 as seen in Table 1 and Fig. 2. In several previous studies, 18:2n-6 was also found to be significantly higher in farmed than in wild fish [27, 29, 30, 34]. However, our group was the first to apply a prospective blinded study design to test the hypothesis that 18:2n-6 could be an accurate marker for determining wild or farmed origin as compared with the current FDA-approved method. In one recent study, Thomas et al. [27] found 18:2n-6 to be the most reliable fatty acid for classification, but their validation procedure also used complex and expensive isotope ratio mass spectrometers. We feel that the mass spec method, which uses several different isotopic mass spectrometers equipped with either chemical analyzers or pyrolysis ovens [27], may be prohibitively complex and expensive. Our data suggests that with this limited sample of fish obtained from North America, 18:2n-6 may be as accurate as using carotenoid chiral isomers, and therefore could be used as the sole identifier of farmed and wild salmon instead of the FDA-approved method. Additionally, the instrumentation for the HPLC analysis would require a capital investment of $30,000, compared to $10,000 for a standard GC. The labor required is also very different. A technician can complete 40 samples per week using fatty acid analysis, as opposed to ten samples per week for the FDA-approved astaxanthin chiral isomer identification test.
With any testing method for salmon, there may be samples that seem ambiguous. In our study, there was one wild sample with 18:2n-6 content of 4.3%, which approached the criterion value of 6.1% (see Fig. 4). The carotenoid profile of this fish was somewhat ambiguous as well. Farmed salmon sometimes escape from their pens and become feral salmon that are caught as wild. One study estimated that the number of escaped farmed salmon could be up to 40% of the total salmon caught as wild in the North Atlantic near the Faroe Islands, a center of fish farming [36]. The number was far lower for catches in the Pacific which averaged below 1%, although in some years higher numbers of escaped farmed salmon caught as wild in the Pacific have been reported [37]. It therefore is reasonable to expect that we could find at least one sample of wild caught salmon that could be a feral Atlantic salmon that escaped, was subsequently caught, and was identified with an intermediate fatty acid profile. Since it takes months for fatty acid and carotenoid profiles to change significantly, escapees may have a fatty acid profile with a ratio between that of typical wild and farmed salmon (see Fig. 4). For example, fish caught near the Faroe Islands might be more difficult to identify by either carotenoid or fatty acid profiles since this area is a center for salmon farming with a high escape rate. Therefore, to ensure consistency and accuracy with our method, continuous monitoring of the fatty acid composition of both cultivated and wild salmon should be considered. If a major change in LNA composition was detected for a given salmon population, the criterion value could be adjusted accordingly. This would eliminate error due to changes in either fishmeal fatty acid content or seasonal variation in wild salmon’s diet. If greater assurances were needed, more sophisticated and time-consuming techniques, such as fish scale identification or multiprobe and multielement isotopic analyses [27, 38], could be employed.
One possible limitation of this study is that we reported each fatty acid as a percentage by weight, instead of the quantitative method using an internal standard to determine the absolute amount of each fatty acid per gram tissue. This study was focused on establishing a rapid, simple and convenient procedure to distinguish farmed from wild salmon, thus the use of the percentage by weight of each fatty acid satisfies these requirements. Some investigators may want to quantify the amount of EPA and DHA in a sample to help identify fish that provide more of these fatty acids in the diet.
To our knowledge, this is the first published study to compare the FDA developed chiral isomer astaxanthin analysis method to the fatty acid analysis by the GC method of identifying farmed and wild salmon. The procedures reported herein are accurate, facile, and may be readily adapted in facilities with capillary GC. For this reason, fatty acid profiles could be of general value for discriminating wild and farmed salmon, with the potential to be applied to other seafood.
Abbreviations
- GC:
-
Gas chromatography
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- HPLC:
-
High performance liquid chromatography
References
Murphy WL, Andersen JM, Ebelin RM (2002) Assessment of geology as it pertains to modeling uplift in jointed rock: a basis for inclusion of uncertainty in flow models. http://libweb.wes.army.mil/uhtbin/hyperion/TR-02-2.pdf
Mozaffarian D, Rimm EB (2006) Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296(15):1885–1899
Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MC, Clay J, Folke C, Lubchenco J, Mooney H, Troell M (2000) Effect of aquaculture on world fish supplies. Nature 405(6790):1017–1024
Pauly D, Watson R, Alder J (2005) Global trends in world fisheries: impacts on marine ecosystems and food security. Philos Trans R Soc Lond B Biol Sci 360(1453):5–12
Fleming IA, Hindar K, Mjolnerod IB, Jonsson B, Balstad T, Lamberg A (2000) Lifetime success and interactions of farm salmon invading a native population. Proc Biol Sci 267(1452):1517–1523
McGinnity P, Prodohl P, Ferguson A, Hynes R, Maoileidigh NO, Baker N, Cotter D, O′Hea B, Cooke D, Rogan G, Taggart J, Cross T (2003) Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc Biol Sci 270(1532):2443–2450
Norford BS (1999) Introduction to papers on the Cambrian-Ordovician boundary. Geol Mag 125(4):323
Hamilton MC, Hites RA, Schwager SJ, Foran JA, Knuth BA, Carpenter DO (2005) Lipid composition and contaminants in farmed and wild salmon. Environ Sci Technol 39(22):8622–8629
Hayward D, Wong J, Krynitsky AJ (2007) Polybrominated diphenyl ethers and polychlorinated biphenyls in commercially wild caught and farm-raised fish fillets in the United States. Environ Res 103(1):46–54
Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ (2004) Global assessment of organic contaminants in farmed salmon. Science 303(5655):226–229
Dewailly E, Ayotte P, Lucas M, Blanchet C (2007) Risk and benefits from consuming salmon and trout: a Canadian perspective. Food Chem Toxicol 45(8):1343–1348
Huang X, Hites RA, Foran JA, Hamilton C, Knuth BA, Schwager SJ, Carpenter DO (2006) Consumption advisories for salmon based on risk of cancer and noncancer health effects. Environ Res 101(2):263–274
Seierstad SL, Seljeflot I, Johansen O, Hansen R, Haugen M, Rosenlund G, Froyland L, Arnesen H (2005) Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur J Clin Invest 35(1):52–59
Torstensen BE, Bell JG, Rosenlund G, Henderson RJ, Graff IE, Tocher DR, Lie O, Sargent JR (2005) Tailoring of Atlantic salmon (Salmo salar L.) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. J Agric Food Chem 53(26):10166–10178
Bell JG, McEvoy J, Tocher DR, McGhee F, Campbell PJ, Sargent JR (2001) Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J Nutr 131(5):1535–1543
Bell JG, Henderson RJ, Tocher DR, McGhee F, Dick JR, Porter A, Smullen RP, Sargent JR (2002) Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J Nutr 132(2):222–230
Miller MR, Nichols PD, Carter CG (2007) Replacement of dietary fish oil for Atlantic salmon parr (Salmo salar L.) with a stearidonic acid containing oil has no effect on omega-3 long-chain polyunsaturated fatty acid concentrations. Comp Biochem Physiol B Biochem Mol Biol 146(2):197–206
Zheng X, Torstensen BE, Tocher DR, Dick JR, Henderson RJ, Bell JG (2005) Environmental and dietary influences on highly unsaturated fatty acid biosynthesis and expression of fatty acyl desaturase and elongase genes in liver of Atlantic salmon (Salmo salar). Biochim Biophys Acta 1734(1):13–24
Bell JG, Henderson RJ, Tocher DR, Sargent JR (2004) Replacement of dietary fish oil with increasing levels of linseed oil: modification of flesh fatty acid compositions in Atlantic salmon (Salmo salar) using a fish oil finishing diet. Lipids 39(3):223–232
Turujman SA, Wamer WG, Wei RR, Albert RH (1997) Rapid liquid chromatographic method to distinguish wild salmon from aquacultured salmon fed synthetic astaxanthin. J AOAC Int 80(3):622–632
Moretti VM, Mentasti T, Bellagamba F, Luzzana U, Caprino F, Turchini GM, Giani I, Valfre F (2006) Determination of astaxanthin stereoisomers and colour attributes in flesh of rainbow trout (Oncorhynchus mykiss) as a tool to distinguish the dietary pigmentation source. Food Addit Contam 23(11):1056–1063
Bell JG, McEvoy J, Webster JL, McGhee F, Millar RM, Sargent JR (1998) Flesh lipid and carotenoid composition of Scottish farmed Atlantic Salmon (Salmo salar). J Agric Food Chem 46(1):119–127
Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18(4):160–167
Abu-Lafi S, Turujman SA (1997) A chiral HPLC method for the simultaneous separation of configurational isomers of the predominant cis/trans forms of astaxanthin. Enantiomer 2(1):17–25
Blalock HM (1979) Social statistics, 2nd edn. McGraw Hill, New York
Thomas DSG, Shaw PA (1999) Late Cenozoic drainage evolution in the Zambezi basin—geomorphological evidence from the Kalahari rim. J Afr Earth Sci Middle East 8(2):40–42
Suountama J, Kiessling A, Melle W, Waagbo R, Olesn RE (2007) Protein from Northern krill (Thysanoessa inermis), Antarctic krill (Euphausia superba) and the Arctic amphipod (Themisto libellula) can partially replace fish meal in diets to Atlantic salmon (Salmo salar) without affecting product quality. Aquac Nutr 13:50–58
Sargent JR, Tacon AG (1999) Development of farmed fish: a nutritionally necessary alternative to meat. Proc Nutr Soc 58(2):377–383
Blanchet C, Lucas M, Julien P, Morin R, Gingras S, Dewailly E (2005) Fatty acid composition of wild and farmed Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Lipids 40(5):529–531
Burros M (2005) Stores say wild salmon, but tests say farm bred. New York Times
Olsen Y, Skjervold H (1995) Variation in content of 3 fatty acids in farmed Atlantic salmon, with special emphasis on effects of non-dietary factors. Aquac Int 3:22–35
Cahu C, Salen P, de Lorgeril M (2004) Farmed and wild-fish in the prevention of cardiovascular diseases: assessing possible differences in lipid nutritional values. Nutr Metab Cardiovasc Dis 14(1):34–41
Nettleton J (2000) Fatty acids in cultivated and wild-fish. In: Proceedings of the International Institute of Fisheries Economics and Trade Oregon State University, Corvallis. http://www.orst.edu/Dept/IIFET/html/i2ktoc.pdf
van Vliet T, Katan MB (1990) Lower ratio of n-3 to n-6 fatty acids in cultured than in wild-fish. Am J Clin Nutr 51(1):1–2
Hansen LP, Jacobsen JA, Lund RA (1999) The incidence of escaped farmed Atlantic salmon, Salmo salar L. in the Faroese fishery and estimates of catches of wild salmon. ICES J Mar Sci 56:200–206
Thomson AJ, Mckinnell S (1996) Recent events concerning Atlantic salmon escapees in the Pacific. J Mar Sci 54:1221–1225
Hansen P, Jacobsen JA, Lund RA (1993) High numbers of farmed Atlantic salmon, Salmo salar, observed in oceanic waters north of the Faroe Islands. Aquac Fisheries Management 24:777–781
Acknowledgments
Lori Megdal is acknowledged for her help on the statistical analysis and manuscript preparation, Edward Dratz for his help with editing this manuscript, and Eugene Rogers for his help with the analysis and identification of fatty acids and GC preparation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Megdal, P.A., Craft, N.A. & Handelman, G.J. A Simplified Method to Distinguish Farmed (Salmo salar) from Wild Salmon: Fatty Acid Ratios Versus Astaxanthin Chiral Isomers. Lipids 44, 569–576 (2009). https://doi.org/10.1007/s11745-009-3294-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-009-3294-6