Abstract
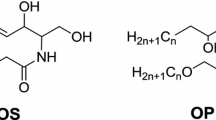
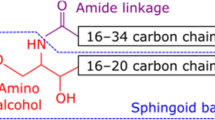
Ceramides are the major type of lipid found in stratum corneum from the skin, gingiva and hard palate. The present study examined the ceramides of the stratum corneum from the hard palate. Six fractions of ceramides were isolated by preparative thin-layer chromatography. The least polar fraction contained an unusual acyl ceramide (EOS) consisting of long ω-hydroxy acids amide-linked to sphingosine with mostly saturated fatty acids ester-linked to the ω-hydroxyl group. The second and third fractions contained normal fatty acids amide-linked to sphingosine (NS) and phytosphingosine (NP), respectively. In each of these ceramides, the fatty acids consisted of a mixture of saturated and monoenoic species. The three most polar fractions all contain amide-linked α-hydroxy acids. The fourth fraction contained long α-hydroxy acids amide-linked to sphingosine (ASl), while the fifth fraction contained short α-hydroxy acids amide-linked to sphingosine (ASs). The most polar ceramide contained α-hydroxy acids amide-linked to phytosphingosine (AP). EOS, NS and NP differed from their epidermal counterparts in terms of the compositions of the normal fatty acids. ASl, ASs and AP from palatal stratum corneum were essentially identical to their epidermal counterparts. The differences between palatal and epidermal EOS, NS and NP contribute to the differences in permeability of palate compared to skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The epidermis covering the skin and the epithelium covering the hard palate are both keratinizing epithelia, and as such, they undergo terminal differentiation to produce a stratum corneum [1]. This consists of an array of flat, keratin-filled cells embedded in a lipid matrix. In both epidermal and oral stratum corneum, ceramides, cholesterol, and fatty acids are major lipid types [2], and the ceramides in both epidermis and hard palatal epithelium consist of the same set of six structural types as judged by thin-layer chromatographic comparison [3]. These lipids determine the permeability of the tissue. In either case, the six chromatographically separable ceramides (EOS, NS, NP, ASl, ASs, AP; the ceramide nomenclature is according to Motta et al. [4] with the addition of the suffixes l or s to indicate long-chain fatty acids or short-chain fatty acids, respectively.) make up about half of the total lipids. The proportions of these major lipids are similar in oral and epidermal stratum corneum, except that there is notably lower ceramide EOS in the oral tissue [3].
Unlike epidermal stratum corneum, palatal and gingival stratum corneum contains small but significant levels of phospholipids and glycolipids [2]. In the epidermis, these polar membrane lipids are completely catabolized in the late stages of the differentiation process leading to formation of the stratum corneum [5]. The oral stratum corneum also contains somewhat variable amounts of triglycerides which are not thought to contribute to barrier function and which may be contaminants [6].
The phospholipids and glycolipids, with their relatively bulky polar head groups and unsaturated fatty acids, would be expected to cause disorder of chain-packing within membranes and thereby shift the balance from predominantly gel-phase membrane domains toward more permeable liquid crystalline domains [7, 8]. In accordance with this expectation, the permeability of oral stratum corneum is an order of magnitude greater than that of epidermal stratum corneum [2, 6].
The purpose of the present study was to determine the structures of the ceramides from porcine palatal stratum corneum and to compare them with the previously published structures of porcine epidermal ceramides [9]. The null hypothesis is that the oral ceramides will be identical in structure to the epidermal ceramides.
Materials and Methods
Stratum Corneum Lipids
Porcine hard palates were obtained at a local slaughter house. The epithelium was peeled off using forceps after 1 min of immersion in water heated to 65 °C [2]. The stratum corneum was isolated by digestion with 1% trypsin in phosphate buffered saline at 4 °C overnight, followed by extensive rinsing with distilled water [2]. The tissue was then frozen at −20 °C and lyophilized over night. During the freeze-drying process, the shelf temperature was kept at 0 °C, and the vacuum was below 13 Pa. In the morning the shelf temperature was raised to 23 °C for 30 min prior to releasing the vacuum.
Lipids were extracted from the dried stratum corneum using chloroform:methanol, 2:1, 1:1 and 1:2 for 2 h each at room temperature [10]. Solvent was removed by evaporation under a gentle stream of nitrogen in a 45 °C water bath.
Isolation of Individual Ceramides
Twenty × twenty centimeter glass plates coated with 0.25-mm-thick silica gel G (Adsorbosil-plus-1; Alltech Associates; Deerfield IL, USA) were washed with chloroform:methanol, 2:1, and activated in an oven at 110 °C. Stratum corneum lipids were dissolved in a small volume of chloroform:methanol, 2:1, and applied as a narrow streak 3 cm from the bottom edge of the plate. The chromatogram was then developed to the top with chloroform:methanol:acetic acid, 190:9:1, followed by hexane:ethyl ether:acetic acid, 70:30:1 [9]. After air drying, the plate was sprayed lightly with 0.01% 2′,7′-dichlorofluorescein in ethanol. After drying again, the plate was viewed under ultraviolet light. Lipid bands appeared as dark bands on a bright background. The lipid fractions of interest were marked using a pencil. The silica from each fraction of interest was scraped from the plate using a Teflon spatula and placed in a small glass column. Lipids were eluted from the silica gel with chloroform:methanol:water, 50:50:1. Solvent was evaporated under nitrogen at 45 °C.
Gas–Liquid Chromatography
Acyl ceramides (EOS) were isolated from both epidermal and palatal stratum corneum from the same pig. The EOS samples were treated with 10% boron trichloride in methanol at 50 °C for 60 min. The normal fatty acid methyl esters (FAME) were isolated by preparative thin-layer chromatography on silica gel with a mobile phase of toluene.
Each of the other ceramides from palatal stratum corneum was treated with 10% boron trichloride at 50 °C overnight to convert the amide-linked fatty acids into FAME, which were isolated by preparative thin-layer chromatography as described above.
The FAME were analyzed by gas–liquid chromatography using a Shimadzu GC-14A gas chromatograph equipped with a 30-m EC-Wax capillary column and a flame ionization detector. This was done isothermally with an oven temperature of 170 °C. The detector and injector temperatures were 250 °C. The standards used included one mixture containing methyl esters of linoleate (C18:2ω6), linolenate (C18:3ω3), arachidonate (C20:4ω6) and docosahexaenoate (C22:6ω3) and a second mixture containing straight-chained saturated FAME (C14:0, C16:0, C18:0, C20:0, C22:0 and C24:0). Fatty acids with chains longer than 24 carbons were identified using plots of log10(retention time) versus carbon number [11]. Such plots are linear for members of a homologous series.
Results
In epidermal stratum corneum the most abundant ester-linked fatty acids from ceramide EOS were linoleic acid (C18:2, 71.7%) and arachidic acid (C20:0, 14.3%). The remainder was mostly palmitic (C16:0) and stearic (C18:) acids with only trace amounts of other components (Table 1). In contrast, the palatal ceramide EOS contained stearic acid (C18:0, 43.6%), palmitic acid (C16:0, 32.0%) as the most abundant species (Table 1). Linoleate was only 8.8% of the ester-linked fatty acid in palatal ceramide EOS. Longer saturated fatty acids (C20:0 and C22:0) were also present.
The compositions of the normal fatty acids from palatal ceramides EOS, NS and NP are given in Table 1. The amide-linked fatty acids from epidermal ceramides NS and NP were completely straight-chained saturated species, mainly C24:0, C26:0 and C28:0. The present findings for the ceramides NS and NP also contain C24:0, C26:0 and C28:0 as the most abundant species, but the presence of abundant monounsaturated species is in striking contrast to the findings with the epidermal ceramides. The compositions of the α-hydroxyacids from ceramides ASl, ASs and AP are listed in Table 2. In contrast with the findings with ceramides NS and NP, the α-hydroxyacids found in palatal ceramides ASl, ASs and AP are essentially identical to those found in their epidermal counterparts. Representative structures of the ceramides from porcine hard palatal stratum corneum are presented in Fig. 1. The long-chain bases were assigned on the basis of chromatographic comparisons with the well characterized epidermal ceramides [2, 3, 9].
Discussion
Although the same structural types of ceramides are present in both oral and epidermal stratum corneum, the present results demonstrate numerous subtle differences in details of these structures. In epidermal ceramide EOS, linoleate is the major ester-linked fatty acid [9]; however, in palatal stratum corneum straight-chained, saturated species prevail. Linoleate in ceramide EOS is essential for the barrier function of the skin [12]. Without a proper skin barrier, transepidermal water loss could lead to dessication, thereby precluding life on dry land [13]. However, in the moist environment of the oral cavity there is no water gradient, and therefore, no need for a barrier to water loss.
As previously reported [9] for porcine epidermal ceramide NS, the major fatty acids in palatal ceramide NS are C24:0, C26:0 and C28:0; however, the presence of significant (23.9% of total) proportions of monounsaturated fatty acids is quite different. The amide-linked fatty acids from epidermal ceramide NS are entirely straight-chained saturated species. Also, palatal ceramide NP has C20:0, C22:0, C24:0, C26:0 and C28:0 as the most abundant amide-linked fatty acids as was the case with epidermal ceramide NP; however, there are again significant levels of monounsaturated fatty acids (7.4% of total) that are not found in epidermal ceramide NP.
Interestingly, the α-hydroxy-acid-containing ceramides (ASl, ASs and AP) are nearly identical to their epidermal counterparts. ASl and ASs are presumed to be produced by hydroxylation of ceramide NS species with long or short fatty chains, respectively, and AP is presumed to be produced by vitamin C-dependent hydroxylation of NP [14]. Present results would be consistent with the suggestion that the α-hydroxylases that mediate these reactions act exclusively on substrates with saturated fatty acyl chains. The possibilities that fatty acids and/or dihydrosphingosine are hydroxylated prior to formation of the amide linkage cannot be ruled out.
As noted in the introduction, the presence of phosphoglycerides and glycosphingolipids in palatal stratum corneum would be expected to have a fluidizing effect on the intercellular membranes by virtue of having bulky polar head groups and some unsaturated acyl chains. The cis double bond in a monounsaturated fatty chain creates a kink in the chain thereby precluding a rod-like geometry and orderly chain-packing within membranes. The unsaturated fatty acids in palatal ceramides NS and NP would further contribute to membrane fluidization and, thereby, reduce barrier function.
One can only speculate about the effects of replacing most of the linoleate in ceramide EOS with saturated fatty acids. With epidermal stratum corneum lipids, it appears that ceramide EOS is required for the assembly of 13-nm trilamellar units [15, 16], and models have been proposed in which the linoleate chains from ceramide EOS are localized in the central lamella of these units [16, 17]. Perhaps the small proportion of linoleate in ceramide EOS is sufficient for formation of such functional units and provision of a barrier function. The barrier requirements of oral mucosa are notably less than the requirements of skin. For one thing, the oral cavity is a wet environment, so there is no need for a water barrier. Perhaps, the EOS species with saturated fatty acids are capable of supporting an adequate barrier function for the oral cavity, and this allows for conservation of an essential nutrient—linoleic acid.
Aside from the roles of the ceramides in the permeability barrier of the stratum corneum, ceramides serve as substrates for ceramidases that liberate antimicrobial long-chain bases near the surface of the stratum corneum, thereby providing a microbial barrier [18].
References
Wertz PW, Squier CA (1996) Biochemical basis of the permeability barrier in skin and oral mucosa. In: Rathbone MJ (ed) Oral mucosal drug delivery. AAI, Wilmington, pp 1–26
Law S, Wertz PW, Swartzendruber DC, Squier CA (1995) Regional variation in content, composition and organization of porcine epithelial barrier lipids revealed by thin-layer chromatography and transmission electron microscopy. Arch Oral Biol 40:1085–1091
Wertz PW, Kremer M, Squier CA (1992) Comparison of lipids from epidermal and palatal stratum corneum. J Invest Dermatol 98:375–378
Motta SM, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R (1993) Ceramide composition of the psoriatic scale. Biochim Biophys Acta 1182:147–151
Yardley HJ, Summerly R (1981) Lipid composition and metabolism in normal and diseased epidermis. Pharmacol Ther 13:357–383
Squier CA, Cox P, Wertz PW (1991) Lipid content and water permeability of skin and oral mucosa. J Invest Dermatol 96:123–126
Forslind B (1994) A domain mosaic model of the skin barrier. Acta Derm Venereol 74:1–6
Wertz PW, van den Bergh BAI (1998) The physical, chemical and functional properties of lipids in the skin and other biological barriers. Chem Phys Lipids 91:85–96
Wertz PW, Downing DT (1983) Ceramides of pig epidermis: structure determination. J Lipid Res 24:759–765
Wertz PW, Downing DT (1983) Acylglucosylceramides of pig epidermis: structure determination. J Lipid Res 24:753–758
Downing DT, Krantz ZH, Murray KE (1960) Studies in waxes. Aust J Chem 13:80–94
Wertz PW (2000) Lipids and barrier function of the skin. Acta Derm Venerol 208:1–5
Attenborough D (1980) Life on earth. Little, Brown & Company, Boston
Ponec M, Weerheim A, Kempenaar J, Mulder A, Gooris GS, Bouwstra JA, Mommaas AM (1997) The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J Invest Dermatol 109:348–355
Kuempel D, Swartzendruber DC, Squier CA, Wertz PW (1998) In vitro reconstitution of stratum corneum lipid lamellae. Biochim Biophys Acta 1372:135–140
Bouwstra JA, Dubbelaar FE, Gooris GS, Ponec M (2000) The lipid organization in the skin barrier. Acta Derm Venereol Suppl 208:23–30
Hill JR, Wertz PW (2003) Molecular models of the intercellular lipid lamellae from epidermal stratum corneum. Biochim Biophys Acta 1616:121–126
Drake DR, Brogden KA, Dawson DV, Wertz PW (2008) Antimicrobial lipids at the skin surface. J Lipid Res 49:4–11
Acknowledgments
This work was supported in part by grants from the US National Institutes of Health (T32DEO 14678, T32DEO 07288 and R01 DEO18032).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hill, J.R., Wertz, P.W. Structures of the Ceramides from Porcine Palatal Stratum Corneum. Lipids 44, 291–295 (2009). https://doi.org/10.1007/s11745-009-3283-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-009-3283-9