Abstract
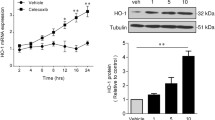
2-Chlorohexadecanal (2-ClHDA), a 16-carbon chain chlorinated fatty aldehyde that is produced by reactive chlorinating species attack of plasmalogens, is elevated in atherosclerotic plaques, infarcted myocardium, and activated leukocytes. We tested the hypothesis that 2-ClHDA and its metabolites, 2-chlorohexadecanoic acid (2-ClHA) and 2-chlorohexadecanol (2-ClHOH), induce COX-2 expression in human coronary artery endothelial cells (HCAEC). COX-2 protein expression increased in response to 2-ClHDA treatments at 8 and 20 h. 2-ClHA also increased COX-2 expression following an 8 h treatment. Quantitative PCR showed that 2-ClHDA treatment increased COX-2 mRNA over 8 h, while 2-ClHA treatment led to a modest increase by 1 h and those levels remained constant over 8 h. 2-ClHDA led to a significant increase in 6-keto-PGF1α release (a measure of PGI2 release) by HCAEC. These data suggest that 2-ClHDA and its metabolite 2-ClHA, which are produced during leukocyte activation, may alter vascular endothelial cell function by upregulation of COX-2 expression.





Similar content being viewed by others
Abbreviations
- RCS:
-
Reactive chlorinating species
- 2-ClHDA:
-
2-Chlorohexadecanal
- 2-ClHA:
-
2-Chlorohexadecanoic acid
- 2-ClHOH:
-
2-Chlorohexadecanol
- HDA:
-
Hexadecanal
- COX-2:
-
Cyclooxygenase-2
- NF-κb:
-
Nuclear factor kappa B
- TNF-α:
-
Tumor necrosis factor-alpha
- HCAEC:
-
Human coronary artery endothelial cells
- MPO:
-
Myeloperoxidase
References
Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P (1999) Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol 155:1281–1291
Masferrer JL, Seibert K, Zweifel B, Needleman P (1992) Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci USA 89:3917–3921
Kraemer SA, Meade EA, DeWitt DL (1992) Prostaglandin endoperoxide synthase gene structure: identification of the transcriptional start site and 5′-flanking regulatory sequences. Arch Biochem Biophys 293:391–400
Whittaker N, Bunting S, Salmon J, Moncada S, Vane JR, Johnson RA, Morton DR, Kinner JH, Gorman RR, McGuire JC, Sun FF (1976) The chemical structure of prostaglandin X (prostacyclin). Prostaglandins 12:915–928
Moncada S, Gryglewski R, Bunting S, Vane JR (1976) An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 263:663–665
Dusting GJ, Moncada S, Vane JR (1977) Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins 13:3–15
Weksler BB, Marcus AJ, Jaffe EA (1977) Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci USA 74:3922–3926
Willems C, van Aken WG (1979) Production of prostacyclin by vascular endothelial cells. Haemostasis 8:266–273
Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM (1993) Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem 268:9049–9054
Daugherty A, Dunn JL, Rateri DL, Heinecke JW (1994) Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94:437–444
Harrison JE, Schultz J (1976) Studies on the chlorinating activity of myeloperoxidase. J Biol Chem 251:1371–1374
Hazen SL, Hsu FF, Duffin K, Heinecke JW (1996) Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J Biol Chem 271:23080–23088
Winterbourn CC, van den Berg JJ, Roitman E, Kuypers FA (1992) Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch Biochem Biophys 296:547–555
Thukkani AK, Hsu FF, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA (2002) Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J Biol Chem 277:3842–3849
Messner MC, Albert CJ, Hsu FF, Ford DA (2006) Selective plasmenylcholine oxidation by hypochlorous acid: formation of lysophosphatidylcholine chlorohydrins. Chem Phys Lipids 144:34–44
Gross RW (1984) High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry 23:158–165
Han X, Gross RW (1994) Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc Natl Acad Sci USA 91:10635–10639
Post JA, Verkleij AJ, Roelofsen B, Op de Kamp JA (1988) Plasmalogen content and distribution in the sarcolemma of cultured neonatal rat myocytes. FEBS Lett 240:78–82
Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA (2001) Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J Biol Chem 276:23733–23741
Thukkani AK, Albert CJ, Wildsmith KR, Messner MC, Martinson BD, Hsu FF, Ford DA (2003) Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J Biol Chem 278:36365–36372
Thukkani AK, McHowat J, Hsu FF, Brennan ML, Hazen SL, Ford DA (2003) Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation 108:3128–3133
Thukkani AK, Martinson BD, Albert CJ, Vogler GA, Ford DA (2005) Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am J Physiol Heart Circ Physiol 288:H2955–H2964
Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, Sattler W, Malle E (2004) 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler Thromb Vasc Biol 24:2302–2306
Wildsmith KR, Albert CJ, Anbukumar DS, Ford DA (2006) Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J Biol Chem 281:16849–16860
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Liu MC, Bleecker ER, Proud D, McLemore TL, Hubbard WC (1988) Profiling of bisenoic prostaglandins and thromboxane B2 in bronchoalveolar fluid from the lower respiratory tract of human subjects by combined capillary gas chromatography-mass spectrometry. Prostaglandins 35:67–79
Schweer H, Kammer J, Seyberth HW (1985) Simultaneous determination of prostanoids in plasma by gas chromatography-negative-ion chemical-ionization mass spectrometry. J Chromatogr 338:273–280
Eligini S, Stella Barbieri S, Cavalca V, Camera M, Brambilla M, De Franceschi M, Tremoli E, Colli S (2005) Diversity and similarity in signaling events leading to rapid Cox-2 induction by tumor necrosis factor-alpha and phorbol ester in human endothelial cells. Cardiovasc Res 65:683–693
Said FA, Werts C, Elalamy I, Couetil JP, Jacquemin C, Hatmi M (2002) TNF-alpha, inefficient by itself, potentiates IL-1beta-induced PGHS-2 expression in human pulmonary microvascular endothelial cells: requirement of NF-kappaB and p38 MAPK pathways. Br J Pharmacol 136:1005–1014
Wu KK (2005) Control of cyclooxygenase-2 transcriptional activation by pro-inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids 72:89–93
Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, Wheeler-Jones CP (2006) Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-kappaB pathways. J Biol Chem 281:11792–11804
Chen CC (2006) Signal transduction pathways of inflammatory gene expressions and therapeutic implications. Curr Pharm Des 12:3497–3508
Duque J, Diaz-Munoz MD, Fresno M, Iniguez MA (2006) Up-regulation of cyclooxygenase-2 by interleukin-1beta in colon carcinoma cells. Cell Signal 18:1262–1269
Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K (2004) A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem 279:48389–48396
Vaillancourt F, Morquette B, Shi Q, Fahmi H, Lavigne P, Di Battista JA, Fernandes JC, Benderdour M (2007) Differential regulation of cyclooxygenase-2 and inducible nitric oxide synthase by 4-hydroxynonenal in human osteoarthritic chondrocytes through ATF-2/CREB-1 transactivation and concomitant inhibition of NF-kappaB signaling cascade. J Cell Biochem 100:1217–1231
Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM (2000) Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J Biol Chem 275:11750–11757
Inoue H, Taba Y, Miwa Y, Yokota C, Miyagi M, Sasaguri T (2002) Transcriptional and posttranscriptional regulation of cyclooxygenase-2 expression by fluid shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol 22:1415–1420
Allport VC, Slater DM, Newton R, Bennett PR (2000) NF-kappaB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH). Mol Hum Reprod 6:561–565
Billack B, Heck DE, Mariano TM, Gardner CR, Sur R, Laskin DL, Laskin JD (2002) Induction of cyclooxygenase-2 by heat shock protein 60 in macrophages and endothelial cells. Am J Physiol Cell Physiol 283:C1267–C1277
Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M (2000) An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem 275:23627–23635
Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T (1995) Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem 270:24965–24971
Kirtikara K, Raghow R, Laulederkind SJ, Goorha S, Kanekura T, Ballou LR (2000) Transcriptional regulation of cyclooxygenase-2 in the human microvascular endothelial cell line, HMEC-1: control by the combinatorial actions of AP2, NF-IL-6 and CRE elements. Mol Cell Biochem 203:41–51
Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T (1994) Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem 221:889–897
Xu Q, Ji YS, Schmedtje JF Jr. (2000) Sp1 increases expression of cyclooxygenase-2 in hypoxic vascular endothelium. Implications for the mechanisms of aortic aneurysm and heart failure. J Biol Chem 275:24583–24589
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Messner, M.C., Albert, C.J. & Ford, D.A. 2-Chlorohexadecanal and 2-Chlorohexadecanoic Acid Induce COX-2 Expression in Human Coronary Artery Endothelial Cells. Lipids 43, 581–588 (2008). https://doi.org/10.1007/s11745-008-3189-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3189-y