Abstract
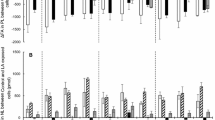
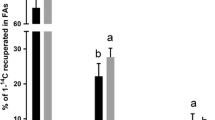
We have studied the effects of dietary FA on the accumulation and secretion of [3H]glycerolipids by salmon hepatocytes in culture. Atlantic salmon were fed diets supplemented with either 100% soybean oil (SO) or 100% fish oil (FO), and grew from an initial weight of 113±5 g to a final weight of 338 ±19 g. Hepatocytes were isolated from both dietary groups and incubated with [3H]glycerol in an FA-free medium; a medium supplemented with 0.75 mM of one of three FA—18∶1n−9, 20∶5n−3, or 22∶6n−3—or a medium supplemented with 0.75 mM of the sulfur-substituted FA analog tetradecylthioacetic acid (TTA), which cannot undergo β-oxidation. Incubations were allowed to proceed for 1,2,6, or 24 h. The rate of the secretion of radioactive glycerolipids with no FA added was 36% lower from hepatocytes isolated from fish fed the FO diet than it was from hepatocytes isolated from fish fed the SO diet. Hepatocytes incubated with 18∶1n−9 secreted more [3H]TAG than when incubated with no FA, whereas hepatocytes incubated with 20∶5n−3 or TTA secreted less labeled TAG than when incubated with no FA. This observation was independent of the feeding group. Hepatocytes incubated with 22∶6n−3 secreted the highest amounts of total [3H]glycerolipids compared with the other treatments, owing to increased secretion of phospholipids and mono- and diacylglycerols (MDG). In contrast, the same amounts of [3H]TAG were secreted from these cells as from cells incubated in an FA-free medium. The lipid-lowering effect of FO is thus independent of 22∶6n−3, showing that 20∶5n−3 is the FA that is responsible for the lipid-lowering effect. The ratio of TAG to MDG in lipids secreted from hepatocytes to which 20∶5n−3 or TTA had been added was lower than that in lipids secreted from hepatocytes incubated with 18∶1n−9 or 22∶6n−3, suggesting that the last step in TAG synthesis was inhibited. Morphometric measurements revealed that hepatocytes incubated with 20∶5n−3 accumulated significantly more cellular lipid than cells treated with 18∶1n−9, 22∶6n−3, TTA, or no treatment. The area occupied by mitochondria was also greater in these cells. The present study shows that dietary FO reduces TAG secretion from salmon hepatocytes and that 20∶5n−3 mediates this effect.
Similar content being viewed by others
Abbreviations
- DGAT:
-
diacylglycerol acyltransferase (EC 2.3.1.20)
- FO:
-
fish oil
- MDG:
-
mono- and diacylglycerol
- PL:
-
phospholipid
- SO:
-
soybean oil
- TTA:
-
tetradecylthioacetic acid
References
Harris, W.S., Connor, W.E., and McMurphy, M.P. (1983) The Comparative Reductions of the Plasma Lipids and Lipoproteins by Dietary Polyunsaturated Fats: Salmon Oil versus Vegetable Oils, Metabolism 32, 179–184.
Nestel, P.J. (1990) Effects of n−3 Fatty Acids on Lipid Metabolism, Annu. Rev. Nutr. 10, 149–167.
Bang, H.O., Dyerberg, J., and Nielsen, A.B. (1971) Plasma Lipid and Lipoprotein Pattern in Greenlandic West-Coast Eskimos, Lancet 1, 1143–1145.
Phillipson, B.E., Rothrock, D.W., Connor, W.E., Harris, W.S., and Illingworth, D.R. (1985) Reduction of Plasma Lipids, Lipoproteins and Apoproteins by Dietary Fish Oils in Patients with Hypertriglyceridemia, N. Engl. J. Med. 312, 1210–1216.
Farrell, A.P. (2002) Coronary Arteriosclerosis in Salmon: Growing Old or Growing Fast? Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 132, 723–735.
Childs, M.T., King, I.B., and Knopp, R.H. (1990) Divergent Lipoprotein Responses to Fish Oils with Various Ratios of Eicosapentaenoic Acid and Docosahexaenoic Acid, Am. J. Clin. Nutr. 52, 632–639.
Rambjor, G.S., Walen, A.I., Windsor, S.L., and Harris, W.S. (1996) Eicosapentaenoic Acid Is Primarily Responsible for the Hypotriglyceridemic Effect of Fish Oil in Humans, Lipids 31, S45-S49.
Otto, D.A., Tsai, C.E., Baltzell, J.K., and Wooten, J.T. (1991) Apparent Inhibition of Hepatic Triacylglycerol Secretion, Independent of Synthesis, in High-Fat Fish Oil-Fed Rats: Role for Insulin, Biochim. Biophys. Acta 1082, 37–48.
Lang, C.A., and Davis, R.A. (1990) Fish Oil Fatty Acids Impair VLDL Assembly and/or Secretion by Cultured Rat Hepatocytes, J. Lipid Res. 31, 2079–2086.
Brown, A.M., Castle, J., Hebbachi, A.M., and Gibbons, G.F. (1999) Administration of n−3 Fatty Acids in the Diets of Rats or Directly to Hepatocyte Cultures Results in Different Effects on Hepatocellular ApoB Metabolism and Secretion, Arterioscler. Thromb. Vasc. Biol. 19, 106–114.
Zheng, X., Avella, M., and Botham, K.M. (2001) Comparison of the Effects of Dietary n−3 and n−6 Polyunsaturated Fatty Acids on Very Low-Density Lipoprotein Secretion When Delivered to Hepatocytes in Chylomicron Remnants, Biochem. J. 357, 481–487.
Haagsman, H.P., de Haas, C.G., Geelen, M.J., and van Golde, L.M. (1982) Regulation of Triacylglycerol Synthesis in the Liver. Modulation of Diacylglycerol Acyltransferase Activity in vitro, J. Biol. Chem. 257, 10593–10598.
Rustan, A.C., Nossen, J.Ø., Christiansen, E.N., and Drevon, C.A. (1988) Eicosapentaenoic Acid Reduces Hepatic Synthesis and Secretion of Triacylglycerol by Decreasing the Activity of Acyl-Coenzyme A:1,2-Diacylglycerol Acyltransferase, J. Lipid Res. 29, 1417–1426.
Berge, R.K., Madsen, L., Vaagenes, H., Tronstad, K.J., Göttlicher, M., and Rustan, A.C. (1999) In Contrast with Docosahexaenoic Acid, Eicosapentaenoic Acid and Hypolipidaemic Derivatives Decrease Hepatic Synthesis and Secretion of Triacylglycerol by Decreased Diacylglycerol Acyltransferase Activity and Stimulation of Fatty Acid Oxidation, Biochem. J. 343, 191–197.
Madsen, L., Rustan, A.C., Vaagenes, H., Berge, K., Dyrøy, E., and Berge, R.K. (1999) Eicosapentaenoic and Docosahexaenoic Acid Affect Mitochondrial and Peroxisomal Fatty Acid Oxidation in Relation to Substrate Preference, Lipids 34, 951–963.
Kendrick, J.S., and Higgins, J.A. (1999) Dietary Fish Oils Inhibit Early Events in the Assembly of Very Low Density Lipoproteins and Target ApoB for Degradation Within the Rough Endoplasmic Reticulum of Hamster Hepatocytes, J. Lipid Res. 40, 504–514.
Frøyland, L., Vaagenes, H., Asiedu, D.K., Garras, A., Lie, Ø., and Berge, R.K. (1996) Chronic Administration of Eicosapentaenoic Acid and Docosahexaenoic Acid as Ethyl Esters Reduced Plasma Cholesterol and Changed the Fatty Acid Composition in Rat Blood and Organs, Lipids 31, 169–178.
Frøyland, L., Madsen, L., Vaagenes, H., Totland, H., Auwerx, J., Kryvi, H., Staels, B., and Berge, R.K. (1997) Mitochondrion Is the Principal Target for Nutritional and Pharmacological Control of Triglyceride Metabolism, J. Lipid Res. 38, 1851–1858.
Nossen, J.Ø., Rustan, A.C., Gloppestad, S.H., Målbakken, S., and Drevon, C.A. (1986) Eicosapentaenoic Acid Inhibits Synthesis and Secretion of Triacylglycerols by Cultured Rat Hepatocytes, Biochim. Biophys. Acta 879, 56–65.
Berge, R.K., Skorve, J., Tronstad, K.J., Berge, K., Gudbrandsen, O.A., and Grav, H. (2002) Metabolic Effects of Thia Fatty Acids, Curr. Opin. Lipidol. 13, 295–304.
Skrede, S., Bremer, J., Berge, R.K., and Rustan, A.C. (1994) Stimulation of Fatty Acid Oxidation by a 3-Thia Fatty Acid Reduces Triacylglycerol Secretion in Cultured Rat Hepatocytes, J. Lipid Res. 35, 1395–1404.
Grisdale-Helland, B., Ruyter, B., Rosenlund, G., Obach, A., Helland, S.J., Sandberg, M.G., Standal, H., and Røsjø, C. (2002) Influence of High Contents of Dietary Soybean Oil on Growth, Feed Utilization, Tissue Fatty Acid Composition, Heart Histology and Standard Oxygen Consumption of Atlantic Salmon (Salmo salar) Raised at Two Temperatures, Aquaculture 207, 311–329.
Seglen, P.O. (1976) Preparation of Isolated Rat Liver Cells, Methods Cell Biol. 1, 29–59.
Dannevig, B.H., and Berg, T. (1985) Endocytosis of Galactose-Terminated Glycoproteins by Isolated Liver Cells of Rainbow Trout, Comp. Biochem. Physiol. 82B, 683–688.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem. 193, 265–275.
Chung, B.H., Wilkinson, T., Geer, J.C., and Segrest, J.P. (1980) Preparative and Quantitative Isolation of Plasma Lipoproteins: Rapid, Single Discontinuous Density Gradient Ultracentrifugation in a Vertical Rotor, J. Lipid Res. 21, 284–291.
Burnette, W.N. (1981) ‘Western Blotting’: Electrophoretic Transfer of Proteins from Sodium Dodecylsulfate-Polyacrylamide Gels to Unmodified Nitrocellulose and Radiographic Detection with Antibody and Radioiodinated Protein A, Anal. Biochem. 112, 195–203.
Folch, J., Lees, M., and Sloane-Stanley, G.M. (1957) A Simple Method for Isolation and Purification of Total Lipids from Animal Tissues, J. Biol. Chem. 226, 497–509.
Ruyter, B., Røsjø, C., Grisdale-Helland, B., Rosenlund, G., Obach, A., and Thomassen, M.S. (2003) Influence of Temperature and High Dietary Linoleic Acid Content on Esterification, Elongation, and Desaturation of PUFA in Atlantic Salmon Hepatocytes, Lipids 38, 833–840.
Hexeberg, S., Frøyland, L., Asiedu, D.K., Demoz, A., and Berge, R.K. (1995) Tetradecylthioacetic Acid Reduces the Amount of Lipid Droplets, Induces Megamitochondria Formation and Increases the Fatty Acid Oxidation in Rat Heart, Mol. Cell. Cardiol. 27, 1851–1857.
SAS (1990) SAS/STAT User’s Guide, SAS Institute, Cary, NC, 1686 pp.
Hardy, R.W., Scott, T.M., and Harrell, L.W. (1987) Replacement of Herring Oil with Menhaden Oil, Soybean Oil, or Tallow in the Diets of Atlantic Salmon Raised in Marine Net-Pens, Aquaculture 65, 267–277.
Thomassen, M.S., and Røsjø, C. (1989) Different Fats in Feed for Salmon: Influence on Sensory Parameters, Growth Rate and Fatty Acids in Muscle and Heart, Aquaculture 79, 129–135.
Greene, D.H.S., and Selivonchick, D.P. (1990) Effects of Dietary Vegetable, Animal and Marine Lipids on Muscle Lipid and Hematology of Rainbow Trout (Oncorhynchus mykiss), Aquaculture 89, 165–182.
Finstad, B., and Thomassen, M.S. (1991) Does Dietary Lipid Composition Affect the Osmoregulatory Ability of Rainbow Trout (Oncorhynchus mykiss) at High and Low Temperatures? Comp. Biochem. Physiol. 99A, 463–471.
Willumsen, N., Vaagenes, H., Lie, Ø,, Rustan, A.C., and Berge, R.K. (1996) Eicosapentaenoic Acid, but Not Docosahexaenoic Acid, Increases Mitochondrial Fatty Acid Oxidation and Upregulates 2,4-Dienol-CoA Reductase Gene Expression in Rats, Lipids 31, 579–592.
Chazan, J.B. (1996) Pharmacological and Dietetic Properties of Oils and Fats, in Oils and Fats Manual, (Karleskind, A., ed.), Vol. 1, pp. 675–702, Intercept Limited, Andover, Hants., United Kingdom.
Sébédio, J.L. (1996) Marine Oils, in Oils and Fats Manual, (Karleskind, A., ed.), Vol. 1, pp. 266–276, Intercept Limited, Andover, Hants., United Kingdom.
Mastellone, I., Polichetti, E., Gres, S., de la Maisonneuve, C., Domingo, N., Marin, V., Lorec, A.M., Farnarier, C., Portugal, H., Kaplanski, G., and Chanussot, F. (2000) Dietary Soybean Phosphatidylcholines Lower Lipidemia: Mechanisms at the Levels of Intestine, Endothelial Cell, and Hepato-biliary Axis, J. Nutr. Biochem. 11, 461–466.
LeBlanc, M.J., Brunet, S., Bouchard, G., Lamireau, T., Yousef, I.M., Gavino, V., Levy, E., and Tuchweber, B. (2003) Effects of Dietary Soybean Lecithin in Rats, J. Nutr. Biochem. 14, 40–48.
Madani, S., Frenoux, J.M., Prost, J., and Belleville, J. (2004) Changes in Serum Lipoprotein Lipids and Their Fatty Acid Compositions and Lipid Peroxidation in Growing Rats Fed Soybean Protein Versus Casein With or Without Cholesterol, Nutrition 20, 554–563.
Aarsland, A., Lundquist, M., Borretsen, B., and Berge, R.K. (1990) On the Effect of Peroxisomal β-Oxidation and Carnitine Palmitoyl-transferase Activity by Eicosapentaenoic Acid in Liver and Heart from Rats, Lipids 25, 546–548.
Martin, L.J., Reaidi, G.B., Gavino, G.R., and Gavino, V.C. (1991) Effect of 4,7,10,13,16,19-Docosahexaenoic Acid on Triglyceride Accumulation and Secretion in Rat Hepatocytes in Culture, Lipids 26, 374–380.
Wang, H.X., Chen, X., and Fisher, E.A. (1993) n−3 Fatty Acids Stimulate Intracellular Degradation of Apoprotein B in Rat Hepatocytes, J. Clin. Invest. 91, 1380–1389.
Botham, K.M., Zheng, X., Napolitano, M., Avella, M., Cavallari, C., Rivabene, R., and Bravo, E. (2003) The Effects of Dietary n−3 Polyunsaturated Fatty Acids Delivered in Chylomicron Remnants on the Transcription of Genes Regulating Synthesis and Secretion of Very Low-Density Lipoprotein by the Liver: Modulation by Cellular Oxidative State, Exp. Biol. Med. 228, 143–151.
Totland, G.K., Madsen, L., Klementsen, B., Vaagenes, H., Kryvi, H., Frøyland, L., Hexeberg, S., and Berge, R. (2000) Proliferation of Mitochondria and Gene Expression of Carnitine Palmitoyltransferase and Fatty Acyl-CoA Oxidase in Rat Skeletal Muscle, Heart and Liver by Hypolipidemic Fatty Acids, Biol. Cell 92, 317–329.
Ruyter, B., and Thomassen, M.S. (1999) Metabolism of n−3 and n−6 Fatty Acids in Atlantic Salmon Liver: Stimulation by Essential Fatty Acid Deficiency, Lipids 34, 1167–1176.
Ruyter, B., Røsjø, C., Einen, O., and Thomassen, M.S. (2000) Essential Fatty Acids in Atlantic Salmon: Time Course of Changes in Fatty Acid Composition of Liver, Blood and Carcass Induced by a Diet Deficient in n−3 and n−6 Fatty Acids, Aquacult. Nutr. 6, 109–117.
Bell, J.G., McVicar, A.H., Park, M.T., and Sargent, J.R. (1991) High Dietary Linoleic Acid Affects the Fatty Acid Compositions of Individual Phospholipids from Tissues of Atlantic Salmon (Salmo salar): Association with Stress Susceptibility and Cardiac Lesion, J. Nutr. 121, 1163–1172.
Bell, J.G., Dick, J.R., McVicar, A.A., Sargent, J.R., and Thompson, K.D. (1993) Dietary Sunflower, Linseed and Fish Oils Affect Phospholipid Fatty Acid Composition, Development of Cardiac Lesions, Phospholipase Activity and Eicosanoid Production in Atlantic Salmon (Salmo salar), Prostaglandins Leukotrienes Essent. Fatty Acids 49, 665–673.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Vegusdal, A., Gjøen, T., Berge, R.K. et al. Effect of 18∶1n−9, 20∶5n−3, and 22∶6n−3 on lipid accumulation and secretion by atlantic salmon hepatocytes. Lipids 40, 477–486 (2005). https://doi.org/10.1007/s11745-005-1407-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-005-1407-z