Abstract
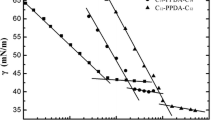
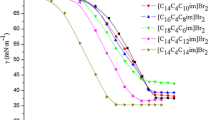
A series of novel dissymmetric gemini surfactants, [C m H2m+1COOC2H4(CH3)2N(CH2)3N(CH3)2C2H4OOCC n H2n+1]Br2 was synthesized and symbolized as m-s–n. The Krafft temperatures and surface tension curves of the dissymmetric gemini surfactants were measured using an electrical conductivity method and a drop volume method. The low Krafft temperatures indicate very good solubility of these esterquat gemini surfactants. With the increasing numbers of carbon atoms in the hydrophobic alkyl chain, the critical micelle concentration (CMC) and the minimum surface area (A min) decrease, and the efficiency of surface tension reduction (pc20) increases. With the same numbers of carbon atoms in the hydrophobic alkyl chain, the dissymmetric gemini surfactant has a lower CMC and a smaller A min than the corresponding symmetric gemini surfactant due to the enhanced hydrophobic interactions.


Similar content being viewed by others
References
Tomokazu Y, Kunio E (2004) Synthesis and surface properties of anionic gemini surfactants with amide groups. J Colloid Interface Sci 276(4):231–238
Kazuyuki T, Yoshiko A, Milton J (2003) Structural effects on surface and micellar properties of alkanediyl-α,ω-bis(sodium N-acyl-β-alaninate) gemini surfactants. J Colloid Interface Sci 262:516–524
Kazuyuki T (2003) The interaction of an anionic gemini surfactant with conventional anionic surfactants. J Colloid Interface Sci 261:524–528
Zana R, Xiao JD (2004) Gemini surfactants. Marcel Dekker, New York
Tehrani-Bagha AR, Holmberg K (2010) Cationic ester-containing gemini surfactants: physical–chemical properties. Langmuir 26:9276–9282
Alami E, Holmberg K (2003) Heterogemini surfactants. Adv Colloid Interface Sci 100–102:13–46
Yoshimura T, Nyuta K, Esumi K (2005) Zwitterionic heterogemini surfactants containing ammonium and carboxylate headgroups. 1. Adsorption and micellization. Langmuir 21(7):2682–2688
Romero FJ, Jimnez C, Huc I (2004) Room temperature synthesis of ordered porous silicas templated by symmetric and dissymmetric gemini surfactants [C m H2m+1(CH3)2N(CH2)2N(CH3)2C n H2n+1]Br2. Microporous Mesoporous Mater 69:43–48
Oda R, Huc I, Candau SJ (1997) Gemini surfactants, the effect of hydrophobic chain length and dissymmetry. Chem Commun 21:2105–2106
Bai G, Wang J, Wang Y, Yan H (2002) Thermodynamics of hydrophobic interaction of dissymmetric gemini surfactants in aqueous solutions. J Phys Chem B 106:6614–6616
Wang XY, Wang JB, Wang YL (2003) Micellization of a series of dissymmetric gemini surfactants in aqueous solution. J Phys Chem B 107:11428–11432
Xu Q, Wang LY, Xing FL (2011) Synthesis and properties of dissymmetric gemini surfactants. J Surfactants Deterg 14:85–90
Sikiric M, Primozic I, Talmon Y, Filipovic-Vincekovic N (2005) Effect of the spacer length on the association and adsorption behavior of dissymmetric gemini surfactants. J Colloid Interface Sci 281:473–481
Wang ZC, Xu HJ, Bao XY (2005) Synthesis and surface active properties of esterquat gemini surfactant. J Jiannan Univ 4:306–309
Banno T, Toshima K, Kawada K, Matsumura S (2009) Synthesis and properties of gemini-type cationic surfactants containing carbonate linkages in the linker moiety directed toward green and sustainable chemistry. J Surfactants Deterg 12:249–259
Nowicki J (2010) Emulsion properties and phase equilibrium and of new asymmetric gemini surfactants consisting of fatty acid ester of polyethoxylated alcohol or phenol. J Surfactants Deterg 13:195–199
Shivaji Sharma K, Hassan PA, Rakshit AK (2006) Self aggregation of binary surfactant mixtures of a cationic dimeric (gemini) surfactant with nonionic surfactants in aqueous medium. Colloid Surf A 289:17–24
Negm NA (2007) Solubilization, surface active and thermodynamic parameters of gemini amphiphiles bearing nonionic hydrophilic spacers. J Surfactants Deterg 10:71–80
Murguía MC, Cristaldi MD, Grau RJ (2008) Synthesis, surface-active properties, and antimicrobial activities of new neutral and cationic trimeric surfactants. J Surfactants Deterg 11:41–48
Abdel-Salam FH, Ei-Said AG (2011) Synthesis and surface active properties of gemini cationic surfactants and interaction with anionic azo dye (AR52). J Surfactants Deterg 14:371–379
Zhao GX, Zhu BY (2003) Principles of surfactant action. China Light Industry Press, Beijing
Rosen MJ (1989) Surfactants and interfacial phenomena. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kang, P., Xu, H. Synthesis and Properties of Dissymmetric Gemini Surfactants. J Surfact Deterg 16, 921–925 (2013). https://doi.org/10.1007/s11743-013-1499-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-013-1499-4