Abstract
A series of novel dissymmetric gemini cationics surfactants was synthesized by three-step reactions. The dissymmetric gemini surfactants contain a dodecanoic acid dimethylethylamine ester as the constant cationic part on one side of the hydroxypropyl center and a similar other cationic part, but with a different acid length (from octanoic to palmitic), on the other side. The critical micelle concentration (CMC) and the effectiveness of surface tension reduction (γ CMC) were determined. The surface tension measurements of dissymmetric gemini surfactants showed good water solubility, and low CMC had great efficiency in lowering the surface tension and a strong adsorption at the air/water interface. The CMC was observed to increase initially with the increase of the ester bond alkyl group. They also showed good foaming properties and wetting capabilites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gemini surfactants consisting of two hydrophobic chains and two polar headgroups covalently linked by a spacer have been attracting much attention [1, 2]. A considerable number of investigations have been carried out on gemini surfactants, focusing on their unique surface and bulk properties, such as high surface activity, low critical micelle concentration (CMC), better wetting properties, unusual viscosity behavior, and specific aggregation structures. Some of the works have been well reviewed by Menger and Zana [3–5]. Because of these unique properties, they have great potential to be used as effective emulsifiers, bactericidal agents, dispersants, anti-foaming agents, and detergents, etc. They are also applicable in the solubilization of dyes and pigments in the textile industry [6] and gene therapy [7]. In a report the surfactants with ester could enhance more effects on biodegradation than long-chain paraffin surfactants, and this will help to improve the environment [8].
In recent years, a new class of gemini surfactants, dissymmetric gemini surfactants that posses two identical head groups but two hydrocarbon chains with different lengths [9–19], appeared and gained much attention. They are in general abbreviated as n-s-m, where s represents the number of carbon atoms in the spacer, and n and m refer to the numbers of carbon atoms in two acids, respectively. Hydrophobic chains with ester bonds of quaternary ammonium gemini surfactants with both the ordinary double-alkyl chains of quaternary ammonium surfactants of high surface activity and with esters bond of quaternary ammonium salt of the biodegradable result in environmentally friendly surfactants [8, 20].
In this work, we synthesized with ester bond dissymmetric gemini surfactants of bis-quaternary ammonium salts by three-step reactions and investigated their physicochemical properties by measuring equilibrium surface tension. In addition, we also compared them with those of symmetric gemini surfactants and discussed the effect of the chain length and the chain numbers on the properties. Scheme 1 shows the synthesis routes of dissymmetric gemini surfactants in this study.
Experimental Procedures
Materials and Equipment Setup
2-Dimethylamino-ethanol, epichlorohydrin (EPIC) and isopropyl alcohol were obtained from Tianjin Chemical Company (China). Ethyl acetate, n-hexane, acetone, methanol, and ethanol were obtained from Beijing Chemical Co. (China). p-Toluene sulphonic acid and hydrochloride acid were obtained from Harbin Chemical Co. (China). Lauric acid, octanoic acid, n-decanoic acid, tetradecanoic acid, and palmitic acid were obtained from Zhejiang Cleaned Chemical Industry Limited Company (China). An FT-IR 20DXB infrared spectrophotometer was used for infrared analyses (Nicolet, American); a DRX 400 nuclear magnetic resonance (NMR) spectrometer was used for 1H NMR analyses (Bruker, 400 MHz, Russ, Germany). Perkin-Elmer 2400II CHNS/O was used for elemental analysis.
Synthesis of a Products
A 1.2-fold molar excess of n-alkyl acid (i.e., octanoic, decanoic, dodecanoic, tetradecanoic, and palmitic acids indicated according to the number of C atoms in the acid, n = 8, 10, 12, 14, 16) was dissolved in ethanol (10 ml) or chloroform (10 ml) and added to 2-dimethylaminoethanol (1.0 mol) containing 3% p-toluenesulfonic. The reaction was heated and continuously stirred at 150 °C for 8–10 h. The residue was washed with ethyl ether several times in order to remove p-toluenesulfonic and unreacted materials, then evaporated. The yellow viscous liquid a was obtained and was used for the synthesis without further purificantion. The synthesis route is the first reaction in Scheme 1.
Synthesis of b Product
A 1.1-fold molar excess of a type with dodecane alkyl group (n = 12) was added to epichlorohydrin (1.0 mol) and hydrochloric acid (0.2 mol) containing methanol (15 ml) and continuously stirred at room temperature for 28–30 h. The solvent and unreacted epichlorohydrin were then evaporated under reduced pressure (12–15 mmHg) at room temperature until a constant mass was achieved. The reaction product was recrystallized from a mixture of hexane and ethyl acetate, and then a mixture of acetone and methanol, or purified by column chromatography (silica gel, 100–200 mesh: eluent: acetone and methol); the corresponding pure compound was dried in a vacuum oven for 6–8 h. The synthesis route of b is shown in Scheme 1, the second reaction.
Synthesis of Gemini Surfactants I
The gemini surfactants were synthesized by reacting b (1.1 mol) with (1.2 mol) of one of the a products (n = 8, 10, 14, 16) in isopropyl alcohol (10 ml) and continuously stirred at 85 °C for 10–12 h, respectively. The synthesis of I is shown as the third reaction in Scheme 1. The crude yellow oil was obtained and frozen in the refrigerator until the crystal precipitated. The crystal was filtered and washed with a cool mixture of hexane and ethyl acetate, and then recrystallized from a mixture of acetone and methanol for at least four to six times till the purity of the compound was confirmed by thin liquid chromatography (TLC). The yield of this reaction step was approximately 39.2%.
IR spectra of all the intermediate a showed absorption bands at 2,925, 2,856 cm−1 (C-H stretching), 1,728 cm−1 (C = O stretching), 1,463 cm−1 (C-H bending), 1,146 cm−1 (C-O stretching), 1,118 cm−1 (C-N stretching), and 720 cm−1 (C-H rocking).
IR spectra of all the intermediate b showed absorption bands at 3,396 cm−1 (−OH stretching), 2,923, 2,855 cm−1 (C-H stretching), 1,725 cm−1 (C = O stretching), 1,461 cm−1 (C-H bending), 1,157 cm−1 (C-O stretching), 1,115 cm−1 (C-N stretching), and 723 cm−1 (C-H rocking).
IR spectra of all the gemini surfactants showed absorption bands at 3,243–3,450 cm−1 (−OH stretching), 2,923, 2,852 cm−1 (C-H stretching), 1,736 cm−1 (C = O stretching), 1,465 cm−1 (C-H bending), 1,166 cm−1 (C-O stretching), 1,110 cm−1 (C-N stretching), and 721 cm−1 (C-H rocking).
1H NMR of the intermediate a (n = 12). 1H NMR (CDCl3) δ 0.88 (t, 3H, −CH3), 1.19–1.25 (m, 18H), 1.69 (m, 2H, CH2CC = O), 2.29 (s, 6H, 2 × N CH3), 2.56 (t, 2H, NCH2), 4.20 (t, 2H, NCCH2-O).
1H NMR of the intermediate b. 1H NMR (CDCl3) δ 0.89 (t, 3H, −CH3), 1.19–1.25 (m, 18H), 1.65 (m, 2H, CH2CC = O), 2.29 (t, 2H, CCH2C = O), 3.32 (s, 6H, 2N+CH3), 3.36–3.55 (t, 6H, N+CH2, and CH2Cl), 4.20 (m, 1H, CH-O), 4.20 (m, 1H, CH-O), 4.55 (t, 2H, N+CCH2).
I-1, represented as (12-S-8). 1H NMR (CDCl3) δ 0.86–0.89 (t, 6H, 2 × CH3), 1.21–1.27 (m, 24H), 1.61–1.73 (m, 4H, 2 × CH2CC = O), 2.29–2.35 (m, 4H, 2 × CH2C = O), 3.28 (s, 12H, 4 × N+CH3), 3.35–3.62 (m, 8H, 4N+CH2), 4.56 (t, 4H, 2NCCH2-O), 4.40–4.51 (m, 1H, CH-O); elemental analysis calculated for C31H64N2O5Cl2; C, 60.47; H, 10.48; N, 4.55%. Found: C, 60.36; H, 10.57; N, 4.56%.
I-2, represented as (12-S-10). 1H NMR (δ, ppm, CDCl3) 0.86–0.89 (t, 6H, 2 × CH3), 1.22–1.25 (m, 28H), 1.61–1.65 (m, 4H, 2 × CH2CC = O), 2.29–2.34 (m, 4H, 2 × CH2C = O), 3.30 (s, 12H, 4 × N+CH3), 3.33–3.60 (m, 8H, 4N+CH2), 4.52 (t, 4H, 2NCCH2-O), 4.43–4.49 (m, 1H, CH-O); elemental analysis calculated for C33H68N2O5Cl2; C, 61.56; H, 10.65; N, 4.35%. Found: C, 61.43; H, 10.72; N, 4.37%.
I-3, represented as (12-S-14). 1H NMR (δ, ppm, CDCl3) 0.86–0.89 (t, 6H, 2 × CH3), 1.22–1.27 (m, 36H), 1.58–1.63 (m, 4H, 2 × CH2CC = O), 2.27–2.33 (m, 4H, 2 × CH2C = O), 3.28 (s, 12H, 4 × N+CH3), 3.31–3.56 (m, 8H, 4N+CH2), 4.55 (t, 4H, 2NCCH2-O), 4.43–4.49 (m, 1H, CH-O); elemental analysis calculated for C37H76N2O5Cl2; C, 70.65; H, 12.18; N, 4.45%. Found: C, 70.38; H, 12.21; N, 4.47%.
I-4, represented as (12-S-16). 1H NMR (δ, ppm, CDCl3) 0.86–0.89 (t, 6H, 2 × CH3), 1.20–1.25 (m, 40H), 1.56–1.65 (m, 4H, 2 × CH2CC = O), 2.26–2.35 (m, 4H, 2 × CH2C = O), 3.26 (s, 12H, 4 × N+CH3), 3.30–3.53 (m, 8H, 4N+CH2), 4.58 (t, 4H, 2NCCH2–O), 4.44–4.50 (m, 1H, CH-O); elemental analysis calculated for C39H80N2O5Cl2; C, 71.29; H, 12.27; N, 4.26%. Found: C, 71.18; H, 12.35; N, 4.32%.
Analytical Methods
Structures of the prepared compounds were confirmed by their spectral data. 1H NMR (400 MHz) spectra were recorded of solutions in CDCl3 using a Bruker DRX 400 spectrometer with tetramethylsilane as a standard. IR spectra were recorded of pellets in KBr using a Nicolet FT-IR 20DXB spectrometer.
Surface tensions of surfactant aqueous solutions at different concentrations were measured at 25 ± 0.1 °C by using a drop-volume tensiometer [21]. The surface tensions were averaged values from three measurements. Accuracy of the surface tension measurements was 0.1 mN/m [22]. Wettability was measured at 25 °C with the canvas descending method [21]. Aqueous surfactant solution containing 2 g/l of the gemini surfactant (from I-1 to I-4) was prepared. Standard thin canvas (diameter 35 mm, weight 38–39 mg) was used, and the detailed operation has been reported [23]. The foaming power was estimated by measuring the foam height of 0.1% aqueous surfactant solution at 5 min after vigorous shaking 100 times at 25 °C with the modified Ross-Miles method [24]. Foam stabilities were determined by comparing the foam height after 5 min to the initial value in the Ross-Miles apparatus [21].
Emulsification properties were investigated by mixing in a graduated cylinder with a standard ground-glass joint and plastic stopper at room temperature (20 °C) [21]. A 20-ml volume of aqueous surfactant solution containing 0.1% of the gemini surfactant was poured into a 100-ml cylinder with 20 ml of solvent (benzene) in the bottom. The stopper was placed in the cylinder, and it was inverted up and down five times successively, then rested for 1 min. The experiment was repeated five times with high repeatability, and the time for the separation of 10 ml water was noted in seconds as indicated in Table 2 [25].
Results and Discussion
Surface Properties
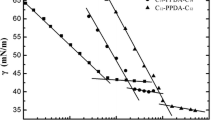
The surface tension (γ) for the water solution-prepared bis-quaternary ammonium salts (I-1, I-2, I-3, I-4) at 25 °C versus log molar concentration (log c) is shown in Fig. 1. The surface tension decreased gradually with increasing surfactant concentration and exhibited a break point, which was taken as the critical micelle concentration (CMC) as indicated in Table 1.
Surface tension (mN.m) versus logarithm of aqueous molar concentration (log C mol/l) of bis-quaternary ammonium salts at 25 °C. See Scheme 1 for compounds
Gemini surfactant structure variation indicates that there is some change with the increasing length of the alkyl group. As n increases (from I-1 to I-4), the CMC increases and the tension also increases at a concentration lower than CMC, and also the minimum tension above the CMC. When compared with the single C16 tail CTAC cationic surfactant results, it is seen that the properties are quite similar, in spite of a very different size of the structure.
The wetting and foaming ability of prepared dissymmetric gemini surfactants measured at 25 °C are summarized in Table 2.
As estimation of wetting power, it is stronger when the time (ability in seconds) is shorter. When surfactants wet the canvas fiber, it will form a three-phase interface of “solid-liquid-gas.” When added to solution, surfactants will adsorb the interface of “solid-liquid” and that of “liquid-gas;” the more surfactants adsorb on the solid surface, the stronger the wetting power is on the canvas. In the molecular structure of bis-quaternary ammonium surfactants, two quaternary ammonium ion head groups are linked by chemical bonds, depending on the linking group containing the hydroxyl group. This structure significantly improves the head group positive density of the bis-quaternary ammonium surfactant unit molecules and the density of the hydrophobic alkyl chain. This makes it easier to be adsorbed on negatively charged surface of the canvas, and the adsorption is more solid, the orientation more closely aligned. The gemini bis-quaternary ammonium surfactant adsorption quantity on the canvas surface is much larger than the corresponding single quaternary ammonium surfactants. And the number of molecules of gemini surfactants with shorter hydrophobic alkyl chains arranged at the interface is more than that of the gemini surfactants with longer hydrophobic alkyl chains. This shows that gemini surfactants with short hydrophobic alkyl chains have stronger wetting power than gemini surfactants with longer hydrophobic alkyl chains. Table 2 shows that the wetting abilities of I-1, I-2, I-3, and I-4 compounds were superior to the corresponding CTAC (179 s) value. In the series the I-1 and I-2 products demonstrated very good wetting power.
The main factors that can affect foamability and stability of surfactants are the interfacial tension and the properties of interfacial film. When generating foam of the same total surface area, a lower surface tension system needs less work. This means that low surface tension is good for foam formation, but also it is good for the foam burst (foam unstable). The stability of the foam mainly depends on the drain speed and intensity of the liquid film, and also on the solution viscosity. Viscosity can increase the strength of liquid film; with higher surface viscosity, liquid does not easily flow and discharge, and the speed of the liquid film thickness to get smaller becomes slower, delaying the damage of liquid film and increasing the stability of foam. Double-layer adsorption is formed in the liquid film surrounding the gas, and hydrophilic groups of surfactants form hydration layer within liquid film. With increasing of the hydrophobic chain length, hydrophobic groups of surfactants attract and tighten, which increases the intensity of the double-layer adsorption film and viscosity of the liquid-in-liquid film leading to stable film formation. The foam-producing ability of all these prepared compounds (from I-1 to I-4) was slightly less than the CTAC value. However, all the compounds exhibited a decrease in the foam height after 5 min. Table 2 shows that by increasing n from 8 to 16, the foam stability become stronger. In addition, all these compounds exhibit a low foam height and are thus good for oilfield water treatment.
Surfactants can prompt emulsion formation and improve its stability. The emulsification is realized by the effect of adsorption, and it forms a layer of adsorptive film in the interface of dispersed droplets, which can prevent or delay collision among droplets and may cause gathering and condensation. The adsorption effect of the oil-water surface in the emulsion O/W is determined by the degree to which hydrophobic groups damage the structure of the water phase, and with the increase in length of the hydrophobic chain, the adsorption effect increases. The emulsifying power of I-4 was found to be the best in the series, and it was even better than that for CTAC shown in Table 2.
Abbreviations
- CTAC:
-
Cetyl trimethyl ammonium chloride
- I-1 or 12-S-8:
-
Dissymetric gemini surfactant with 12 and 8 alkyl acid groups
- I-2 or 12-S-10:
-
Dissymetric gemini surfactant with 12 and 10 alkyl acid groups
- I-3 or 12-S-14:
-
Dissymetric gemini surfactant with 12 and 14 alkyl acid groups
- I-4 or 12-S-16:
-
Dissymetric gemini surfactant with 12 and 16 alkyl acid groups
References
Menger FM, Littau CA (1991) Gemini-surfactants: synthesis properties. J Am Chem Soc 113:1451–1452
Menger FM, Littau CA (1991) Gemini surfactants: a new class of self-assembling molecules. J Am Chem Soc 115:10083–10090
Menger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Ed 39:1906–1920
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interf Sci 97:203–251
Zana R (2002) Dimeric (Gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interf Sci 248:203–220
Idouhar M, Tazerouti A (2008) Spectrophotometric determination of cationic surfactants using patent blue V: application to the wastewater industry in Algiers. J Surfact Deterg 11:263–267
Alami E, Beinert G, Marie P, Zana R (1993) Alkanediyl-alpha-omega-bis (dimethyl-alkylammonium bromide) surfactants behavior at the air-water interface. Langmuir 9:1465–1467
Banno T, Toshima K, Kawada K, Matsumura S (2009) Synthesis and properties of gemini-type cationic surfactants containing carbonate linkages in the linker moiety directed toward green and sustainable chemistry. J Surfact Deterg 12:249–259
Oda R, Huc I, Candau SJ (1997) Gemini surfactants, the effect of hydrophobic chain length and dissymmetry. Chem Commun 21:2105–2106
Zhang J, Zheng Y, Yu P, He L, Wang H, Wang R (2010) Synthesis, characterization and surface-activity of a polyoxyethylene ether trimeric quaternary ammonium surfactant. J Surfact Deterg 13:155–158
Nowicki J (2010) Emulsion properties and phase equilibrium and of new asymmetric gemini surfactants consisting of fatty acid esters of polyethoxylated alcohol or phenol. J Surfact Deterg 13:195–199
Oda R, Huc I, Danino D, Talmon Y (2000) Aggregation properties and mixing behavior of hydrocarbon, fluorocarbon, and hybrid hydrocarbon-fluorocarbon cationic dimeric surfactants. Langmuir 16:9759–9769
Romero FJ, César J, Huc I, Oda R (2004) Room temperature synthesis of ordered porous silicas templated by symmetric and dissymmetric gemini surfactants [CnH2n+1N(CH3)2(CH2)2(CH3)2NCmH2m+1]Br2. Micropor Mesopor Mater 69:43–48
Sikirić M, Ljerka I, Tušek-Božić L, Tomašić V, Pucić I, Primozić I, Filipović-Vinceković N (2003) Effect of the spacer length on the solid phase transitions of dissymmetric gemini surfactants. Langmuir 19:10044–10053
Sikirić M, Primožić I, Talmon Y, Filipović-Vinceković N (2002) Adsorption and association in aqueous solutions of dissymmetric gemini surfactant. J Colloid Interf Sci 250:221–229
Sikirić M, Primožić I, Talmon Y, Filipović-Vinceković N (2005) Effect of the spacer length on the association and adsorption behavior of dissymmetric gemini surfactants. J Colloid Interf Sci 281:473–481
Li ZX, Dong CC, Wang JB, Thomas RK (2002) Unusual surface structure in layers of cationic gemini surfactants adsorbed at the air/water interface: a neutron reflection study. Langmuir 18:6614–6622
Bai G, Wang J, Wang Y, Yan H (2002) Thermodynamics of hydrophobic interaction of dissymmetric gemini surfactants in aqueous solutions. J Phys Chem B 106:6614–6616
Sun Y, Dong H, Feng Y, Chen Z (2006) Synthesis and surface activity properties of a series of cationic gemini surfactants. Acta Chim Sinica 64:1925–1928
Talmage Syliva S (2004) Environmental and human safety of major surfactant. Lewis Publishers, Boca Raton
Mao P (1988) Synthetic detergent industrial analysis. Peking: the publishing house of light industry. pp 456–462
Zhang D, Fan W, Zhao M (2001) Quick calculation of determining surface tension in drop volume method. China Surfact Deterg Cosemet 1:49–50
Li S, Ding L (2003) Study on the wettability of APG complex. J Anhui Univ Sci Technol (Nat Sci) 4:43–45
Karuna M, Devi B, Prasad P, Prasad R (2009) Synthesis of sulfated sodium salts of 1-alkylamino-3-alkyloxy-2-propanols and N, N-di-(2-hydroxy-3-alkyloxy propyl) alkylamines as potential surfactants. J Surfact Deterg 12:117–123
Jia W, Rao X, Song Z, Shang S (2009) Microwave-assisted synthesis and properties of a novel cationic gemini surfactant with the hydrophenanthrene structure. J Surfact Deterg 12:261–267
Kim TS, Kida T, Nakatsuji Y, Ikeda I (1996) Surface-active properties of novel cationic surfactants with two alkyl chains and two ammonium groups. J Am Oil Chem Soc 73:907–911
Acknowledgments
We are grateful for the financial support from the Studying Abroad Fund Program of the Office of Science and Technology of the Heilongjiang Province, China (project no. LC08C32).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Xu, Q., Wang, L. & Xing, F. Synthesis and Properties of Dissymmetric Gemini Surfactants. J Surfact Deterg 14, 85–90 (2011). https://doi.org/10.1007/s11743-010-1207-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-010-1207-6