Abstract
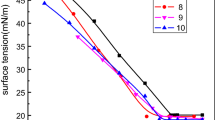
Two environmentally friendly succinic acid monofluoroalkyl sulfonate surfactants were synthesized from maleic anhydride and polyethylene glycol mono (1H,1H,7H-dodecafluoroheptyl) ether, i.e. H(CF2)6CH2OCH2CH2OCOCH(SO3Na)CH2COOH (FEOS-1) and H(CF2)6CH2(OCH2CH2)3OCOCH(SO3Na)CH2COOH (FEOS-3). The obtained surfactants were characterized by FT-IR, 1H NMR, 13C NMR and 19F NMR in detail. The synthesized fluorinated surfactants have a high thermal stability on the basis of thermogravimetric analysis. Their surface properties were examined and the results show that FEOS-1 and FEOS-3 surfactants can reduce the surface tension of water to 25.55 mN m−1 at 10.25 mmol L−1 and 21.63 mN m−1 at 8.33 mmol L−1, respectively; meanwhile, the introduction of oxyethylene groups enhances the hydrophilicity and micellar forming ability and the longer oxyethylene chains the better surface properties. The Krafft points (K p) of FEOS-1 and FEOS-3 were both below 0 °C, which was lower than perfluoro-n-heptanesulfonic acid sodium salt (n-C7F15SO3Na, K p = 56.5 °C) at a similar length of fluorocarbon chains. Comparison studies on two surfactants above and the conventional fluorocarbon surfactants, perfluorooctanoate of ammonium (PFOA) show that the surfactants have comparable properties to PFOA, thus offering an environmentally friendly synthesizing alternatives to PFOA.










Similar content being viewed by others
References
Abe M (1999) Synthesis and applications of surfactants containing fluorine. Curr Opin Colloid Interface Sci 4:354–356
Shinoda K, Hato M, Hayashi T (1972) Physicochemical properties of aqueous solutions of fluorinated surfactants. J Phys Chem 76:909–914
Yoshino N, Yamamoto Y, Hamano K, Kawase T (1993) Syntheses and reactions of metal organics. XVIII. Syntheses of (1H,1H,2H,2H-Polyfluoroalkyl)trimethoxysilanes and surface modification of glass plate. Bull Chem Soc Jpn 66:1754–1758
Yoshino N, Yamamoto Y, Seto T, Tominaga S, Kawase T (1993) Syntheses and reactions of metal organics. XVII. Synthesis of silane coupling agent having fluorocarbon chain and surface modification of glass plate. Bull Chem Soc Jpn 66:472–476
Yoshino N, Yamamoto Y, Teranaka T (1993) Surface modification of denture to provide contamination-free ability by using silane coupling agent containing fluorocarbon chain. Chem Lett 22:821–824
Kunitake T, Tawaki S, Nakashima N (1983) Excimer formation and phase separation of hydrocarbon and fluorocarbon bilayer membranes. Bull Chem Soc Jpn 56:3235–3242
Kim CU, Lee JM, Ihm SK (1999) Emulsion polymerization of tetrafluoroethylene: effects of reaction conditions on the polymerization rate and polymer molecular weight. J Appl Polym Sci 73:777–793
Badr MZ, Birnbaum LS (2004) Enhanced potential for oxidative stress in livers of senescent rats by the peroxisome proliferator-activated receptor alpha agonist perfluorooctanoic acid. Mech Ageing Dev 125:69–75
Kawashima Y, Suzuki S, Kozuka H, Sato M, Suzuki Y (1994) Effects of prolonged administration of perfluorooctanoic acid on hepatic activities of enzymes which detoxify peroxide and xenobiotic in the rat. Toxicology 93:85–97
Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP (2002) Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ Sci Technol 36:3210–3216
Yang BQ, Chen K, Xing H, Xiao JX (2009) Perfluorobutyl-based fluorinated surfactant with high surface activity. Acta Phys Chim Sin 25:2409–2412
Taylor CK (1999) Fluorinated surfactants in practice. Annu Surfactants Rev 2:271–316
Krafft MP, Riess JG (2007) Perfluorocarbons, life sciences and biomedical uses. J Polym Sci A Polym Chem 45:1185–1198
Kostov G, Boschet F, Ameduri F (2009) Original fluorinated surfactants potentially non-bioaccumulable. J Fluorine Chem 130:1192–1199
Guittard F, Geribaldi S (2001) Highly fluorinated molecular organised systems: strategy and concept. J Fluorine Chem 107:363–374
Vierling P, Santaella C, Greiner J (2001) Highly fluorinated amphiphiles as drug and gene carrier and delivery systems. J Fluorine Chem 107:337–354
Ohno A, Kushiyama A, Kondo Y, Kondo Y, Teranaka T, Yoshino N (2008) Synthesis and properties of gemini-type hydrocarbon–fluorocarbon hybrid surfactants. J Fluorine Chem 129:577–582
Guo W, Li Z, Fung BM, O’Rear EA, Harwell JH (1992) Hybrid surfactants containing separate hydrocarbon and fluorocarbon chains. J Phys Chem 96:6738–6742
Yoshino N, Hamano K, Omiya Y, Kondo Y, Ito A, Abe M (1995) Syntheses of hybrid anionic surfactants containing fluorocarbon and hydrocarbon chains. Langmuir 11:466–469
Miyazawa H, Kondo Y, Yoshino N (2005) Synthesis and solution properties of sulfate-type hybrid surfactants with an ethylene spacer. J Oleo Sci 54:167–178
Kondo Y, Yokochi E, Mizumura S, Yoshino N (1998) Syntheses of novel fluorocarbon surfactants with oxyethylene groups. J Fluorine Chem 91:147–151
Wang Q, Zhang SX, Geng B, Zhang LQ, Zhao JQ, Shi JH (2012) Synthesis and surface activities of novel monofluoroalkyl phosphate surfactants. J Surfactants Deterg 15:83–88
Rosen MJ (2004) Surfactants and interfacial phenomena. Wiley, New York
Pabon M, Corpart JM (2002) Fluorinated surfactants: synthesis, properties, effluent treatment. J Fluorine Chem 114:149–156
Krafft MP, Riess JG (2009) Chemistry, physical chemistry, and uses of molecular fluorocarbon–hydrocarbon diblocks, triblocks, and related compounds unique “apolar” components for self-assembled colloid and interface engineering. Chem Rev 109:1714–1792
Xiao JX, Zhao ZG (2003) Application principle of surfactants. Chemical Industry Press, Beijing
Liang ZQ, Chen P (1998) Fluorinated surfactant. China Light Industry Press, Beijing
Acknowledgments
This project was financially supported by the National Natural Science Foundation of China (No. 20774037).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhang, L., Shi, J., Xu, A. et al. Synthesis and Surface Activities of Novel Succinic Acid Monofluoroalkyl Sulfonate Surfactants. J Surfact Deterg 16, 183–190 (2013). https://doi.org/10.1007/s11743-012-1366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1366-8