Abstract
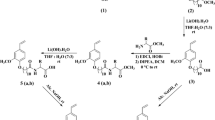
The present paper describes the synthesis and evaluation of surface properties of a novel series of anionic surfactant, namely sodium 3-(3-alkyloxy-3-oxopropoxy)-3-oxopropane-1-sulfonate with varying alkyl chain length (C8–C16). Synthesis involves initial formation of the 3-alkyloxy-3-oxopropyl acrylate along with fatty acrylate during the direct esterification of fatty alcohol with acrylic acid in the presence of 0.5 % NaHSO4 at 110 °C followed by sulfonation of the terminal double bond of the 3-alkyloxy-3-oxopropyl acrylate. Synthesized compounds were evaluated for surface and thermodynamic properties such as critical micelle concentration (CMC), surface tension at CMC (γcmc), efficiency of surface adsorption (pC20), surface excess (Γmax), minimum area per molecule at the air–water interface (A min), free energy of adsorption (∆G°ads), free energy of micellization (∆G°mic), wetting time, emulsifying properties, foaming power and calcium tolerance. Effect of chain length on CMC follows the classic trend, i.e. decrease in CMC with the increase in alkyl chain length. High pC20 (>3) value indicates higher hydrophobic character of the surfactant. These surfactants showed very poor wetting time and calcium tolerance, but exhibited good emulsion stability and excellent foamability. Foaming power and foam stability of C14-sulfonate were found to be the best among the studied compounds. Foam stability of C14-sulfonate was also studied at different concentrations over time and excellent foam stability was obtained at a concentration of 0.075 %. Thus this novel class of surfactant may find applications as foam boosters in combination with other suitable surfactants.




Similar content being viewed by others
References
Bauer W (1987) Acrylic acid and derivatives. In: Kirk-Othmer encyclopedia of chemical technology, 4th edn. Wiley, New York, vol 1, p 287
Rehberg CE, Fisher CH (1944) Preparation and properties of the n-alkyl acrylates. J Am Chem Soc 66:1203–1207
Lal J, Green R (1955) The preparation of some esters of methacrylic acid. J Org Chem 20:1030–1033
Kautter CT, Baumann U (1969) Esterification of acrylic acid. US Patent 3,458,561
Sogabe H, Takeda T (1999) Process for production of carboxylic acid ester and resin-separating vessel used therein. US Patent 5892103
Sert E, Atalay FS (2012) Esterification of acrylic acid with different alcohols catalyzed by zirconia supported tungstophosphoric acid. Ind Eng Chem Res 51:6666–6671
Chen X, Xu Z, Okuhara T (1999) Liquid phase esterification of acrylic acid with 1-butanol catalyzed by solid acid catalysts. App Cat A 180:261–269
Yang J, Cho S-H, Park J, Lee K-Y (2007) Esterification of acrylic acid with 1,4-butanediol in a batch distillation column reactor over Amberlyst 15 catalyst. Can J Chem Eng 85:883–888
Dupont P, Védrine JC, Paumard E, Hecquet G, Lefebvre F (1995) Heteropolyacids supported on activated carbon as catalysts for the esterification of acrylic acid and butanol. App Cat A 129:217–227
Gassama A, Ernenwein C, Youssef A, Agach M, Riguet E, Marinkovic S, Estrine B, Hoffmann N (2013) Sulfonated surfactants obtained from furfural. Green Chem 15:1558–1566
Zocchi G (1999) Handbook of detergents, part A—surfactant properties. In: Broze G (ed) Surfactant science series, vol 82. Marcel Dekker, New York, pp 424–427
Subrahmanyam VVR, Achaya KT (1961) Structure and surfactant evaluation of ricinoleyl alcohol. J Chem Eng Data 6:38
Indian Standard No. 1185 (1957) Bureau of Indian Standards, New Delhi
Wilkes BG, Wickert JN (1937) Synthetic aliphatic penetrants. Ind Eng Chem 29:1234–1239
Ostrowski S, Jamroz ME, Cz Dobrowollski J (2011) Formation of heavy adducts in esterification of acrylic acid: a DFT study. Comp Theor Chem 974:100–108
Sreenu M, Nayak RR, Prasad RBN, Sreedhar B (2014) Synthesis, surface and micellar properties of N-oleoyl amino acid. Coll Surf A 449:74–81
Kanjilal S, Maiti S, Kaimal TNB (1999) Synthesis and physicochemical studies of methyl-12-[1-β-d-lactosyl]-octadec-9-ene-1-oate: a biosurfactant analog. J Surf Det 2:531–538
Mukherjee P (1967) The nature of the association equilibria and hydrophobic bonding in aqueous solutions of association colloids. Adv Colloid Interface Sci 1:242–275
Moulik SP, Haque ME, Jana PK, Das AR (1996) Micellar properties of cationic surfactants in pure and mixed states. J Phys Chem 100:701–708
Acknowledgments
A Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India to PPK is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kumar, P.P., Nayak, R.R. & Kanjilal, S. Synthesis and Surface Properties of a Novel Sodium 3-(3-Alkyloxy-3-oxopropoxy)-3-oxopropane-1-sulfonate at the Air–Water Interface. J Surfact Deterg 18, 689–695 (2015). https://doi.org/10.1007/s11743-015-1691-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1691-9