Abstract
The prior studies have shown that interleukin-2 (IL-2) exerts important roles in the pathological and physiological processes of lung diseases. However, the role of IL-2 in community-acquired pneumonia (CAP) is still uncertain. Through a prospective cohort study, our research will explore the correlations between serum IL-2 levels and the severity and prognosis in CAP patients. There were 267 CAP patients included. Blood samples were obtained. Serum IL-2 were tested by enzyme-linked immunosorbent assay (ELISA). Demographic traits and clinical characteristics were extracted. Serum IL-2 were gradually elevated with increasing severity scores in CAP patients. Correlation analyses revealed that serum IL-2 were connected with physiological parameters including liver and renal function in CAP patients. According to a logistic regression analysis, serum IL-2 were positively correlated with CAP severity scores. We also tracked the prognostic outcomes of CAP patients. The increased risks of adversely prognostic outcomes, including mechanical ventilation, vasoactive agent usage, ICU admission, death, and longer hospital length, were associated with higher levels of IL-2 at admission. Serum IL-2 at admission were positively associated with severe conditions and poor prognosis among CAP patients, indicated that IL-2 may involve in the initiation and development of CAP. As a result, serum IL-2 may be an available biomarker to guide clinicians in assessing the severity and determining the prognosis of CAP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Community-acquired pneumonia (CAP) is a condition characterized by inflammation of the pulmonary tissue resulting from an infection contracted outside of hospital settings, namely within the community. Additionally, it ranks among the leading causes of death globally. Although it caused a large number of deaths, it is still not considered by the public as an issue that needs attention [1,2,3,4,5]. For CAP, the physician can diagnose by the main clinical manifestations and ancillary tests, but in many patients, the presentation of pneumonia may be atypical non-respiratory symptoms such as fatigue, muscle aches, diarrhea, etc. [3]. These atypical pneumonias may be overlooked by clinicians. Although the clinically diagnostic criteria and severity scores can guide the diagnosis and determine the severity of CAP, the subjectivity of clinical symptoms and prolonged pathogen cultivation may sometimes hinder accurate assessment of the severity and prognosis of CAP timely. Therefore, in order to reduce the progression of mild pneumonia to severe pneumonia, as well as the mortality, a characteristic biomarker is needed to aid in early assessment.
Interleukin-2 (IL-2), a common cytokine, which is mainly produced in the human body by helper T cells 1, and it possesses the capability to facilitate cellular growth and differentiation, as well as the secretion of cytokines, and directly or indirectly participates in cellular and humoral immune regulation [6]. The previous researches have indicated that numerous inflammation-mediated disorders, including Crohn's disease, autoimmune arthritis, obesity-related metabolic inflammation, etc., have been related with IL-2. [7,8,9,10,11]. Recent surveys have identified the correlations between IL-2 and several pulmonary diseases [12,13,14,15,16,17,18].
Though IL-2 plays a significant role in pulmonary diseases, it is unclear whether IL-2 is associated with CAP. Previous studies have shown that there are large numbers of recruited neutrophils in the lungs of pneumonia patients [19]. IL-2 can bind to the interleukin-2 receptor β and γ chains on human neutrophils to induce pulmonary edema [20, 21]. Therefore, we hypothesized that IL-2 may participate in the pathophysiologic process of CAP. This prospective cohort study sought to explore the correlations between serum IL-2 levels and the severity as well as the prognosis of CAP patients.
Methods
Subjects
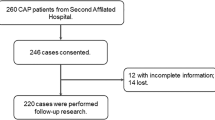
From August 2021 to September 2022, this research was performed in the Second Affiliated Hospital of Anhui Medical University. Every patient fulfilled the following diagnostic criteria: (1) occurred in the community; (2) older than 18 years of age; (3) new or progressive infiltrative lesions of the lungs; (4) met one of the following symptoms: temperature > 38℃, respiratory symptoms such as coughing and sputum, signs of solid changes in the lungs on percussion and wet rales on auscultation, elevated white blood cell count [22, 23]. The exclusion criteria were: (1) pregnant women; (2) hospitalized for more than 2 weeks within 3 months due to illness; (3) treatment with intravenous antibiotics, antiviral drugs, glucocorticoid in the last week; (4) accompanied with other respiratory diseases such as lung malignancy, COPD, bronchiectasis; (5) accompanied with autoimmune diseases [24,25,26]. All 288 hospitalized patients who agreed to participate in this study were recruited. Twelve serum samples were lost and 9 patients with incomplete information were later excluded and there were 267 hospitalized patients enrolled in total (Supplemental Fig. 1). Additionally, in order to evaluate the level of IL-2 in healthy volunteers, healthy participators from the Physical Examination Center of the Second Affiliated Hospital of Anhui Medical University were collected. Every control subject was matched with one CAP patients in accordance with age and sex. The control groups were without respiratory system diseases such as CAP, COPD, asthma, lung cancers, and etc. Lastly, 267 eligible control subjects were selected and enrolled. Demographic characteristics and clinical information were gathered from the electronic medical record system. The related prognostic outcomes were tracked-up. The primary outcomes was mortality, the secondary outcomes were ICU admission, mechanical ventilation, vasoactive drugs and longer hospital stays among CAP inpatients. Before starting to use any antibiotics, serum samples were collected from each patient. Routine blood and routine biochemical tests were performed. In order to assess the extent of pneumonia severity, various severity scores have been utilized.
Serum IL-2 levels in CAP patients with different severity. The levels of serum IL-2 were measured through ELISA. A Serum IL-2 levels in healthy individuals and CAP patients. B–F The levels of serum IL-2 in CAP cases with different severity scores. B CURB-65 score. C SMART-COP score. D CRB-65 score. E PSI score. F APACHE II score. *P < 0.05, **P < 0.01
Enzyme-linked immunosorbent assay (ELISA)
Fasting blood was collected and centrifuged at 6 a.m. on the second day after admission. Serum IL-2 were detected via ELISA at a unified time. IL-2 (CSB-E04626h) ELISA kits were from Cusabio in Wuhan, China. (https://www.cusabio.com/). All ELISA techniques were carried out in accordance with the earlier studies [27, 28].
Statistical analysis
Demographic characteristics and laboratory parameters were represented by the median or mean. Categorical variables are represented by frequencies (percentages). To examine differences in characteristics between groupings, ANOVA or chi-square tests were employed. The relationships between clinical parameters and serum IL-2 were investigated using Spearman or Pearson correlative analyses and linear regression models. Linear and logistic regression models were utilized to determine the correlation between the levels of serum IL-2 and the severity of CAP. The association between prognostic outcomes and serum IL-2 was investigated using mixed logistic regression models. The presence of statistical significance was observed when the P-value was found to be less than 0.05 in the conducted research.
Results
Demographic and clinical information
Among all CAP patients, the median respiratory rate was 19.8 breaths per minute and the median oxygen saturation (room air) was 94.8% (Table 1). CAP patients were divided into three groups based on the tertiles of serum IL-2: tertile 1 (T1) with IL-2 below 5.86 pg/mL, tertile 2 (T2) with IL-2 ranging from 5.86 to 7.23 pg/mL, and tertile 3 (T3) with IL-2 above 7.23 pg/mL. As appeared in Table 1, of these, 157 were men, accounting for 58.8%. The median BMI was 22.3 kg/m2. In addition, the mean of systolic blood pressure was 125.2 mmHg, the average of diastolic blood pressures was 75.2 mmHg. Moreover, microbiological diagnosis was analyzed. Among all CAP patients, 108 (40.4%) cases were infected with streptococcus pneumonia, 13 (4.9%) cases with staphylococcus aureus, 18 (6.7%) cases with legionella pneumophila, 44 (16.1%) cases with other atypical pathogens. Moreover, 10 (3.7%) subjects with respiratory virus, 9 (3.4%) subjects with pseudomonas aeruginosa, 6 (2.2%) subjects with enterobacteriaceae, and 60 (22.5%) with other etiologies. Our study also analyzed the comorbid conditions in patients with CAP. In Table 1, 72 patients had hypertension (27.0%), 30 patients had diabetes mellitus (11.2%), 26 patients had the past history of cerebral infarction (9.7%), 15 patients had coronary heart disease (5.6%), and only 4 cases had chronic bronchitis (1.5%). Some serological markers were measured in all patients, such as procalcitonin (PCT), D- dimer, tumor necrosis factor-α (TNF-a), C-reaction protein (CRP), interleukin-6 (IL-6), etc. There was no distinction in age, gender, BMI, heart rate, blood pressure, body temperature, hypertension, diabetes mellitus, coronary artery disease, and bronchitis among CAP patients in three groups. Whereas the respiratory rate, PCT, D-dimer, TNF-α, and IL-6 increased with rising serum IL-2, and oxygen saturation decreased with growing IL-2 (Table 1). In addition, the CAP severity scores went up consistently as the increased serum IL-2 levels (Table 1).
IL-2 levels in CAP patients of various severity
In contrast to the serum IL-2 levels observed in healthy individuals, the serum IL-2 levels were significantly elevated in CAP patients (Fig. 1A). The levels of IL-2 were compared among CAP patients with various severities. Based on the CURB-65 score, serum IL-2 levels were substantially lower in group 0–1 and 2 than these in group 3–5 (Fig. 1B). According to SMART-COP score, an obvious rise of IL-2 in group 5–6 and 7–8 compared to CAP patients with the group 0–2 and 3–4 scores, and the serum IL-2 levels were much greater in group 7–8 than these in the other groups (Fig. 1C). In the CRB-65 score, the IL-2 levels of the group ≥ 3 scores were higher than that of the group in 0 and the 1–2 scores (Fig. 1D). According to the PSI score, IL-2 gradually increased with the PSI score, with the most pronounced increase in the group V (Fig. 1E). On the basis of APACEH II score, the levels of IL-2 in the group > 9 scores were markedly elevated than the group 4–6 and 6–9 scores (Fig. 1F).
Relationships between serum IL-2 and clinical features
In this project, the correlation between serum IL-2 levels and blood routine, liver, and kidney function were evaluated in CAP patients. The data demonstrated a positive correlation between serum IL-2 and white blood cell (WBC) (r = 0.13, P < 0.05), a negative relationship between serum IL-2 with lymphocyte (r = -0.19, P < 0.01) and eosinophil (r = -0.14, P < 0.05). However, there was no meaningful relationship between the level of IL-2 and neutrophil, monocyte, and basophil counts. Additionally, serum IL-2 levels were strongly correlated with aspartate aminotransferase (AST) (r = 0.25, P < 0.001), alanine aminotransferase (ALT) (r = 0.25, P < 0.001), urea nitrogen (r = 0.17, P < 0.01), creatinine (r = 0.3, P < 0.001), and lactate dehydrogenase (LDH) (r = 0.28, P < 0.001) levels. Moreover, serum IL-2 was positively correlated with D-dimer (r = 0.23, P < 0.001), PCT (r = 0.20, P < 0.05) (Fig. 2). The mixed linear regression model showed that each 1 pg/mL increase in serum IL-2 was negatively associated with lymphocyte (β = -0.012; 95% CI: -0.021 ~ -0.003) and eosinophil (β = -0.002; 95% CI: -0.004 ~ 0.000). In addition, serum IL-2 were positively correlated with creatinine (β = 1.217; 95% CI: 0.634 ~ 1.800), ALT (β = 5.451; 95% CI: 2.868 ~ 8.034), AST (β = 15.373; 95% CI: 8.090 ~ 22.657), LDH (β = 10.213; 95% CI: 4.789 ~ 15.637), PCT (β = 0.122; 95% CI: 0.026 ~ 0.219), D-dimer (β = 0.049; 95% CI: 0.015 ~ 0.083), IL-6 (β = 6.812; 95% CI: 3.029 ~ 10.595) among CAP patients (Supplemental Table 1).
Relationships between serum IL-2 and clinical characteristics in CAP patients. The correlations between serum IL-2 and clinical characteristics were examined by Spearman or Pearson correlative analysis. The top half values indicated the degree of correlation strength. Red color indicates the positive correlation, and blue color indicates the negative correlation. The darker the color, the stronger the correlation. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Relationships between serum IL-2 and CAP severity scores
The mixed linear and logistic regression models were adjusted for age, hypertension, diabetes mellitus, cerebral infarction, coronary heart disease, and bronchitis. The mixed linear regression model found that each 1 pg/mL increase in serum IL-2 was associated with 0.028 scores (95% CI: 0.016 ~ 0.041), 0.040 scores (95% CI: 0.025 ~ 0.054), 0.094 scores (95% CI: 0.068 ~ 0.120), and 0.357 scores (95% CI: 0.284 ~ 0.431) increases for CRB-65, CURB-65, SMART-COP, and APACHE II, respectively (Table 2). In addition, mixed logistic regression models were performed. Compared to the lowest IL-2 group (T1), the highest IL-2 group (T3) in CAP patients showed a 6.650-fold increase in CRB-65, a 4.860-fold increase in CURB-65, a 2.644-fold increase in SMART-COP, and a 1.989-fold increase in APACHE II. The above results displayed the positive correlations between serum IL-2 levels and the CAP severity scores (Table 2).
Relationships between serum IL-2 and prognostic outcomes
Throughout the duration of hospitalization, poor prognosis was carefully observed and meticulously tracked in CAP patients (Table 3). Compared with T1 group, mechanical ventilation was observed in 31 patients (34.8%) (RR = 7.753; 95% CI: 2.940 ~ 20.447), 17 patients (19.1%) received vasoactive agent (RR = 8.983; 95% CI: 1.757 ~ 20.372), there were 33 patients (37.1%) with ICU admission (RR = 7.576; 95% CI: 2.858 ~ 20.085), 18 patients dead in-hospital (20.2%) (RR = 13.137; 95% CI: 2.685 ~ 64.267), and 35 patients (39.3%) with longer hospital stays (RR = 3.708; 95% CI: 1.719 ~ 7.995) in T3 group (Table 3). The numbers of the poor prognosis in CAP patients were much higher in T3 group compared to the other groups. These results showed that an elevated serum IL-2 on admission was positively associated with an increased risk of mortality in-hospital, ICU admission, mechanical ventilation, vasoactive drugs and length of hospitalization in CAP patients.
The predictive capacities for severity and death between serum IL-2 and clinical characteristics
The estimation of the predictive ability for severity and mortality were conducted through the receiver operating characteristic (ROC) area under the curve (AUC). The predicted capacities for severities were as follows: CURB-65, 0.898; CRB-65, 0.915; PSI, 0.842; SMART-COP, 0.947; APACHE II, 0.842; IL-2, 0.702; IL-6, 0.663; IL-2 + CRB-65, 0.706; IL-2 + SMART-COP, 0.708; IL-2 + CURB-65, 0.707; IL-2 + PSI, 0.858; IL-2 + APACHE II, 0.755 (Fig. 3A). The cut-off concentration of serum IL-2 was 10.40 pg/mL. The precision of the assessment was determined, exhibiting a sensitivity of 69% and a specificity of 97%. In addition, the capability of predicting mortality was also examined. The levels of predictability were as follows: serum IL-2, 0.817; CRB-65, 0.858; SMART-COP, 0.944; CURB-65, 0.865; APACHE II, 0.849; PSI, 0.831; IL-2 + CRB-65, 0.817; IL-2 + SMART-COP, 0.819; IL-2 + CURB-65, 0.818; IL-2 + PSI, 0.844; IL-2 + APACHE II, 0.840; IL-6, 0.580 (Fig. 3B). The threshold of serum IL-2 for mortality was 8.50 pg/mL, exhibiting a specificity of 82% and a sensitivity of 90%.
Discussion
This prospective cohort study was aimed to explore the relationships between serum IL-2 levels with the severity and prognosis of CAP patients. The key findings included: (1) there were the positive correlations between the CAP severity scores and the levels of serum IL-2; (2) the risk of poor prognosis was positively associated with the elevated serum IL-2 levels on admission in CAP patients; (3) serum IL-2 had a certain predictive ability for the severity and mortality of CAP.
In the human organism, IL-2 is predominantly released by activated T lymphocytes, while dendritic cells and macrophages also possess the capacity to secrete limited quantities of IL-2 [29]. The effects of IL-2 are contingent upon its dosage and affinity for receptors, leading to a diverse range of outcomes [30,31,32,33]. Published research studies have shown that IL-2 is strongly connected to the biological processes of many lung diseases. In an experimental model utilizing mice, IL-2 is significantly elevated and can exacerbate allergic asthma through the toll-like receptor 9-IL-2 axis [34]. Patients with COVID-19 who are undergoing cytokine storm demonstrate elevated serum levels of IL-2, which serves as an immunopathologic attribute [35,36,37]. In addition, IL-2 can synergistically promote vascular leakage with TNF-α in superantigen or pathogen-induced acute lung injury [20, 38]. However, the exact connection between IL-2 and CAP has not yet been identified. Therefore, IL-2 levels were examined in all CAP patients. Based on our study, we discovered that patients with CAP had gradually rising serum IL-2 levels as their severity scores rose. Furthermore, serum IL-2 levels on admission were strongly connected to CAP severity scores. Additionally, various forms of organ impairment were found in CAP patients [22,23,24,25,26]. Therefore, we estimated the relationships between serum IL-2 and various clinical parameters. The facts from our study revealed that serum IL-2 were positively correlated with WBCs, other inflammatory factors, and indices related to hepatic and renal functions, such as urea nitrogen, creatinine, AST, ALT. The implications of these findings propose that IL-2 may play a pivotal role in the pathophysiological mechanisms of CAP.
Numerous investigations have substantiated the findings that IL-2 is involved in the progression of many other diseases and associated with its prognosis. High expression of IL-2 is positively correlated with the survival period of multiple myeloma patients [39]. The presence of IL-2 exacerbates the poor prognosis of colorectal cancer patients [40]. When IL-2 is elevated in patients diagnosed with non-Hodgkin lymphoma, their survival rates are diminished [41]. But the relationship between IL-2 and the prognosis was unclear in CAP patients. Our research revealed that the increased IL-2 levels on admission elevated the risks of the mortality in-hospital, ICU admission, mechanical ventilation, vasoactive drugs, and longer hospital stays in CAP patients. The predictive abilities of serum IL-2, other inflammatory markers, and severity scores for severity and mortality were assessed through ROC curve. The results indicated that IL-2 had a certain predictive ability for the severity and mortality of CAP. Although the predictive capacities of serum IL-2 for severity and death were slightly weaker than those in CAP severity scores system. They were obviously elevated compared with commonly inflammatory cytokines, such as IL-6. Even so, serum IL-2 combination with CAP severity scores can’t elevated the predive powers for severity and death compared with single serum IL-2 or CAP severity scores among CAP patients. These results suggested that IL-2 is implicated in the pathophysiological processes of CAP. It is possible that IL-2 inhibition can decelerate the progression of illness in CAP. Therefore, serum IL-2 level may be used as a candidate biomarker for guiding clinical practice, including illness estimate, prognose analysis, and therapeutic targets. Thus, the results from our study demonstrated that higher levels of serum IL-2 on admission were associated with an increased risk of poor prognosis in CAP patients.
IL-2 is recognized to be a common pro-inflammatory factor that is widely expressed in human immune cells [29]. CAP can be caused by various pathogenic infections, mainly included gram-positive bacteria, gram-negative bacteria, viruses and other atypical pathogens [42]. Gram-negative bacterial cell membranes contain lipopolysaccharide. When it binds to TLR4 expressed on airway epithelial cells [43], it activates the NF-κB transcription factor, causing pro-inflammatory cytokines like IL-2, IL-4, IL-5, and IL-6 to be released [44]. Additionally, SARS-CoV-2 infection can activate NF-κB, resulting in a "cytokine storm" that releases plenty of inflammatory factors, including IL-2, which in turn leads to lung damage in COVID-19 patients [45]. High IL-2 concentration can induce vascular leakage syndrome and cytokine storms, leading to interstitial pulmonary edema and multi-organ failure [46,47,48]. An animal experiment indicated that Streptococcus pneumonia infection incurs pulmonary inflammation and IL-2 elevation in the lungs [49]. Therefore, we speculate that pathogenic infection may activate NF-κB signaling and evoke inflammatory cytokines secretion, leading to IL-2 elevation in lungs. Then, IL-2 is secreted, and the levels of serum IL-2 are elevated in CAP patients.
This research endeavored to illuminate the role of IL-2 in CAP. It predominantly demonstrates the positive correlations between serum IL-2 concentration on admission and the severity as well as unfavorable prognosis of CAP patients. Nevertheless, the present investigation possessed certain limitations. First, as a result of the limited sample size at present, future research necessitates larger sample sizes and the inclusion of multiple centers. Second, only serum samples were utilized to assess IL-2 levels. Further investigation is warranted to assess the levels of IL-2 in pulmonary tissues and bronchoalveolar lavage fluid. Third, the current study only was an epidemiological examination of the population. The mechanism underlying the enhancement in IL-2 remained unidentified in CAP patients. Animal experiments are required to perform and explore the exact mechanism.
In the previous studies, CAP patients are enrolled from inpatient department. Actually, most CAP patients are treated and cured in the outpatient department [50]. Due to the disease condition, there is no need to be hospitalized for the majority of CAP cases. So, the conclusions are extrapolated form inpatients to outpatients. This is the current research status. Mounting evidence have revealed that mild CAP patients can be treated and cured in the outpatient department. The mortality rate is very low (0.5%), and it is evidently lower than those in the current research (7.49%). Moreover, the risk of poorly prognostic outcomes is infrequent among CAP patients [51]. Therefore, we think it doesn’t affect the validity of the extrapolation of the results. Of course, this is a research defect. To improve the reliability of conclusions, more confirmed research will be conducted in the outpatients.
Conclusions
Through a prospective cohort analysis, this research verified the relationships between serum IL-2 and the severity and prognostic outcomes of CAP patients. We discovered that serum IL-2 levels were gradually increased with elevated CAP severity scores. There were strong associations between serum IL-2 levels and clinical parameter in CAP patients. In addition, Serum IL-2 were positively related to CAP severity scores and poor outcomes. All conclusions suggest that IL-2 may involve in the pathophysiological process of CAP. Therefore, in forthcoming clinical practice, the potential of serum IL-2 as a valuable biomarker for assessing the severity and prognosis is anticipated to be explored in CAP patients.
Availability of data and materials
The corresponding author can provide all the data and information of this research.
References
Arshad H, Alfonso JCL, Franke R, Michaelis K, Araujo L, Habib A, Zboromyrska Y, Lücke E, Strungaru E, Akmatov MK, Hatzikirou H, Meyer-Hermann M, Petersmann A, Nauck M, Brönstrup M, Bilitewski U, Abel L, Sievers J, Vila J, Illig T, Schreiber J, Pessler F (2019) Decreased plasma phospholipid concentrations and increased acid sphingomyelinase activity are accurate biomarkers for community-acquired pneumonia. J Transl Med 17(1):365. https://doi.org/10.1186/s12967-019-2112-z
Khan F, Owens MB, Restrepo M, Povoa P, Martin-Loeches I (2017) Tools for outcome prediction in patients with community acquired pneumonia. Expert Rev Clin Pharmacol 10(2):201–211. https://doi.org/10.1080/17512433.2017.1268051
Lanks CW, Musani AI, Hsia DW (2019) Community-acquired pneumonia and hospital-acquired pneumonia. Med Clin North Am 103(3):487–501. https://doi.org/10.1016/j.mcna.2018.12.008
Ferreira-Coimbra J, Sarda C, Rello J (2020) Burden of community-acquired pneumonia and unmet clinical needs. Adv Ther 37(4):1302–1318. https://doi.org/10.1007/s12325-020-01248-7
Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI (2021) Community-acquired pneumonia. Lancet 398(10303):906–919. https://doi.org/10.1016/s0140-6736(21)00630-9
Alapati T, Sagal KM, Gudiseva HV, Pistilli M, Pyfer M, Chavali VRM, O’Brien JM (2021) Evaluating TNF-α and Interleukin-2 (IL-2) levels in African American primary open-angle glaucoma patients. Genes (Basel) 13(1):54. https://doi.org/10.3390/genes13010054
Thornton S, Boivin GP, Kim KN, Finkelman FD, Hirsch R (2000) Heterogeneous effects of IL-2 on collagen-induced arthritis. J Immunol 165(3):1557–1563. https://doi.org/10.4049/jimmunol.165.3.1557
Ebrahimpour S, Shahbazi M, Khalili A, Tahoori MT, Zavaran Hosseini A, Amari A, Aghili B, Abediankenari S, Mohammadizad H, Mohammadnia-Afrouzi M (2017) Elevated levels of IL-2 and IL-21 produced by CD4+ T cells in inflammatory bowel disease. J Biol Regul Homeost Agents 31(2):279–287
Katz LH, Kopylov U, Fudim E, Yavzori M, Picard O, Ungar B, Eliakim R, Ben-Horin S, Chowers Y (2014) Expression of IL-2, IL-17 and TNF-alpha in patients with Crohn’s disease treated with anti-TNF antibodies. Clin Res Hepatol Gastroenterol 38(4):491–498. https://doi.org/10.1016/j.clinre.2014.01.010
Tong L, Zhang X, Hao H, Liu Q, Zhou Z, Liang X, Liu T, Gong P, Zhang L, Zhai Z, Hao Y, Yi H (2021) Lactobacillus rhamnosus GG derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in DSS-induced colitis mice. Nutrients 13(10):3319. https://doi.org/10.3390/nu13103319
Kochumon S, Al Madhoun A, Al-Rashed F, Thomas R, Sindhu S, Al-Ozairi E, Al-Mulla F, Ahmad R (2020) Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci Rep 10(1):16364. https://doi.org/10.1038/s41598-020-73347-y
Mat Z, Grensemann B, Yakin Y, Knobloch J, Koch A (2012) Effect of lipoteichoic acid on IL-2 and IL-5 release from T lymphocytes in asthma and COPD. Int Immunopharmacol 13(3):284–291. https://doi.org/10.1016/j.intimp.2012.04.005
Knobloch J, Chikosi SJ, Yanik S, Rupp J, Jungck D, Koch A (2016) A systemic defect in Toll-like receptor 4 signaling increases lipopolysaccharide-induced suppression of IL-2-dependent T-cell proliferation in COPD. Am J Physiol Lung Cell Mol Physiol 310(1):L24-39. https://doi.org/10.1152/ajplung.00367.2014
Bożek A, Rogala B (2018) IgE-dependent sensitization in patients with COPD. Ann Agric Environ Med. 25(3):417–420. https://doi.org/10.26444/aaem/83413
Chyczewska E, Mróz RM, Kowal E (1997) IL-2 concentration in bronchoalveolar lavage fluid (BALF) of non-small cell lung cancer (NSCLC) patients. Rocz Akad Med Bialymst 42(Suppl 1):136–145
Ju ST, Sharma R, Gaskin F, Fu SM (2012) IL-2 controls trafficking receptor gene expression and Th2 response for skin and lung inflammation. Clin Immunol 145(1):82–88. https://doi.org/10.1016/j.clim.2012.07.015
Krieg C, Létourneau S, Pantaleo G, Boyman O (2010) Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 107(26):11906–11911. https://doi.org/10.1073/pnas.1002569107
Krakauer T (2019) Staphylococcal superantigens: pyrogenic toxins induce toxic shock. Toxins (Basel) 11(3):178. https://doi.org/10.3390/toxins11030178
Domon H, Terao Y (2021) The role of neutrophils and neutrophil elastase in pneumococcal pneumonia. Front Cell Infect Microbiol 11:615959. https://doi.org/10.3389/fcimb.2021.615959
Huzella LM, Buckley MJ, Alves DA, Stiles BG, Krakauer T (2009) Central roles for IL-2 and MCP-1 following intranasal exposure to SEB: a new mouse model. Res Vet Sci 86(2):241–247. https://doi.org/10.1016/j.rvsc.2008.07.020
Li J, Gyorffy S, Lee S, Kwok CS (1996) Effect of recombinant human interleukin 2 on neutrophil adherence to endothelial cells in vitro. Inflammation 20(4):361–372. https://doi.org/10.1007/bf01486739
Hua DX, Ma KS, Cheng JY, Liu Y, Sun J, He QY, Deng YP, Yang J, Fu L, Zhao H (2022) Serum TRAIL predicts severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. Intern Emerg Med 17(8):2279–2290. https://doi.org/10.1007/s11739-022-03086-7
Feng CM, Wang XM, Li MD, Xu Z, Hua DX, Cheng JY, Zheng L, Zhao H, Fu L (2021) Serum interleukin-17 predicts severity and prognosis in patients with community acquired pneumonia: a prospective cohort study. BMC Pulm Med 21(1):393. https://doi.org/10.1186/s12890-021-01770-6
Liu HY, Xiang HX, Xiang Y, Xu Z, Feng CM, Fei J, Fu L, Zhao H (2021) The associations of serum S100A9 with the severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. BMC Infect Dis 21(1):327. https://doi.org/10.1186/s12879-021-06020-y
Xu Z, Wang XM, Cao P, Zhang C, Feng CM, Zheng L, Xu DX, Fu L, Zhao H (2022) Serum IL-27 predicts the severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. Int J Med Sci 19(1):74–81. https://doi.org/10.7150/ijms.67028
Xu Z, Hou XF, Feng CM, Zheng L, Xu DX, Zhao H, Fu L (2023) The association between serum complement C3a and severity in patients with community-acquired pneumonia. Front Immunol 14:1034233. https://doi.org/10.3389/fimmu.2023.1034233
Li W, Zhao X, Yu TT, Hao W, Wang GG (2021) Knockout of PKC θ gene attenuates oleic acid-induced acute lung injury via reduction of inflammation and oxidative stress. Iran J Basic Med Sci 24(7):986–991. https://doi.org/10.22038/ijbms.2021.56908.12695
Pu Z, Wang W, Xie H, Wang W (2024) Apolipoprotein C3 (ApoC3) facilitates NLRP3 mediated pyroptosis of macrophages through mitochondrial damage by accelerating of the interaction between SCIMP and SYK pathway in acute lung injury. Int Immunopharmacol 128:111537. https://doi.org/10.1016/j.intimp.2024.111537
Létourneau S, Krieg C, Pantaleo G, Boyman O (2009) IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol 123(4):758–762. https://doi.org/10.1016/j.jaci.2009.02.011
Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12(3):180–190. https://doi.org/10.1038/nri3156
de Picciotto S, DeVita N, Hsiao CJ, Honan C, Tse SW, Nguyen M, Ferrari JD, Zheng W, Wipke BT, Huang E (2022) Selective activation and expansion of regulatory T cells using lipid encapsulated mRNA encoding a long-acting IL-2 mutein. Nat Commun 13(1):3866. https://doi.org/10.1038/s41467-022-31130-9
Heiler S, Lötscher J, Kreuzaler M, Rolink J, Rolink A (2018) Prophylactic and therapeutic effects of interleukin-2 (IL-2)/anti-IL-2 complexes in systemic lupus erythematosus-like chronic graft-versus-host disease. Front Immunol 9:656. https://doi.org/10.3389/fimmu.2018.00656
Yuan Y, Kolios AGA, Liu Y, Zhang B, Li H, Tsokos GC, Zhang X (2022) Therapeutic potential of interleukin-2 in autoimmune diseases. Trends Mol Med 28(7):596–612. https://doi.org/10.1016/j.molmed.2022.04.010
Murakami Y, Ishii T, Nunokawa H, Kurata K, Narita T, Yamashita N (2020) TLR9-IL-2 axis exacerbates allergic asthma by preventing IL-17A hyperproduction. Sci Rep 10(1):18110. https://doi.org/10.1038/s41598-020-75153-y
Patterson BK, Guevara-Coto J, Yogendra R, Francisco EB, Long E, Pise A, Rodrigues H, Parikh P, Mora J, Mora-Rodríguez RA (2021) Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front Immunol 12:700782. https://doi.org/10.3389/fimmu.2021.700782
Li X, Qiu Q, Li M, Lin H, Cao S, Wang Q, Chen Z, Jiang W, Zhang W, Huang Y, Luo H, Luo L (2021) Chemical composition and pharmacological mechanism of ephedra-glycyrrhiza drug pair against coronavirus disease 2019 (COVID-19). Aging (Albany NY) 13(4):4811–4830. https://doi.org/10.18632/aging.202622
Yuan Y, Wang QP, Sun D, Wu ZB, Peng H, Liu XW, Liu YL (2021) Differences in immune responses between children and adults with COVID-19. Curr Med Sci 41(1):58–61. https://doi.org/10.1007/s11596-021-2318-1
Dubinett SM, Huang M, Lichtenstein A, McBride WH, Wang J, Markovitz G, Kelley D, Grody WW, Mintz LE, Dhanani S (1994) Tumor necrosis factor-alpha plays a central role in interleukin-2-induced pulmonary vascular leak and lymphocyte accumulation. Cell Immunol 157(1):170–180. https://doi.org/10.1006/cimm.1994.1214
Botta C, Di Martino MT, Ciliberto D, Cucè M, Correale P, Rossi M, Tagliaferri P, Tassone P (2016) A gene expression inflammatory signature specifically predicts multiple myeloma evolution and patients survival. Blood Cancer J 6(12):e511. https://doi.org/10.1038/bcj.2016.118
Taylor ES, McCall JL, Shen S, Girardin A, Munro FM, Black MA, Ward-Hartstonge KA, Kemp RA (2018) Prognostic roles for IL-2-producing and CD69(+) T cell subsets in colorectal cancer patients. Int J Cancer 143(8):2008–2016. https://doi.org/10.1002/ijc.31598
Ozdemir F, Aydin F, Yilmaz M, Kavgaci H, Bektas O, Yavuz MN, Yavuz AA (2004) The effects of IL-2, IL-6 and IL-10 levels on prognosis in patients with aggressive Non-Hodgkin’s Lymphoma (NHL). J Exp Clin Cancer Res 23(3):485–488
Zha L, Li X, Ren Z, Zhang D, Zou Y, Pan L, Li S, Chen S, Tefsen B (2022) Pragmatic comparison of piperacillin/tazobactam versus carbapenems in treating patients with nosocomial pneumonia caused by extended-spectrum β-lactamase-producing klebsiella pneumoniae. Antibiotics (Basel). 11(10):1384. https://doi.org/10.3390/antibiotics11101384
Athari SS, Athari SM, Beyzay F, Movassaghi M, Mortaz E, Taghavi M (2017) Critical role of Toll-like receptors in pathophysiology of allergic asthma. Eur J Pharmacol 808:21–27. https://doi.org/10.1016/j.ejphar.2016.11.047
Wu Z, Mehrabi Nasab E, Arora P, Athari SS (2022) Study effect of probiotics and prebiotics on treatment of OVA-LPS-induced of allergic asthma inflammation and pneumonia by regulating the TLR4/NF-kB signaling pathway. J Transl Med 20(1):130. https://doi.org/10.1186/s12967-022-03337-3
Pu Z, Sui B, Wang X, Wang W, Li L, Xie H (2023) The effects and mechanisms of the anti-COVID-19 traditional Chinese medicine, dehydroandrographolide from andrographis paniculata (Burm.f.) wall, on acute lung injury by the inhibition of NLRP3-mediated pyroptosis. Phytomedicine 114:154753. https://doi.org/10.1016/j.phymed.2023.154753
Assier E, Jullien V, Lefort J, Moreau JL, Di Santo JP, Vargaftig BB, Lapa e Silva JR, Thèze J (2004) NK cells and polymorphonuclear neutrophils are both critical for IL-2-induced pulmonary vascular leak syndrome. J Immunol 172(12):7661–8. https://doi.org/10.4049/jimmunol.172.12.7661
Schwartz RN, Stover L, Dutcher JP (2002) Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 16(11 Suppl 13):11–20
Wu R, Li N, Zhao X, Ding T, Xue H, Gao C, Li X, Wang C (2020) Low-dose interleukin-2: biology and therapeutic prospects in rheumatoid arthritis. Autoimmun Rev 19(10):102645. https://doi.org/10.1016/j.autrev.2020.102645
Feng J, Dai W, Zhang C, Chen H, Chen Z, Chen Y, Pan Q, Zhou Y (2020) Shen-ling-bai-zhu-san ameliorates inflammation and lung injury by increasing the gut microbiota in the murine model of Streptococcus pneumonia-induced pneumonia. BMC Complement Med Ther 20(1):159. https://doi.org/10.1186/s12906-020-02958-9
Froes F, Pereira JG, Póvoa P (2018) Outpatient management of community-acquired pneumonia. Curr Opin Infect Dis 31(2):170–176. https://doi.org/10.1097/qco.0000000000000435
Cillóniz C, Ewig S, Polverino E, Marcos MA, Prina E, Sellares J, Ferrer M, Ortega M, Gabarrús A, Mensa J, Torres A (2012) Community-acquired pneumonia in outpatients: aetiology and outcomes. Eur Respir J 40(4):931–938. https://doi.org/10.1183/09031936.00168811
Funding
This study was funded by National Natural Science Foundation of China (82100078 and 82270071), University Natural Science Research Project of Anhui Province (2023AH030117), the Major Scientific Research Project of the Department of Education of Anhui Province (2022AH040098), and the Research Foundation of Anhui Medical University (2022xkj170).
Author information
Authors and Affiliations
Contributions
Conceptualization: LF, JY, HZ; methodology: F-MZ, JX, Q-YH, Y-PD, M-YL, YL and JS; data curation: Q-YH, Y-PD, M-YL, YL and JS; formal analysis and investigation: F-MZ, JX; writing—original draft preparation: F-MZ; writing—review and editing: F-MZ, JX, YL and JS; supervision: LF, JY.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
This research has obtained approval from the Ethics Committee of Second Affiliated Hospital of Anhui Medical University (YJ-YX2021-147).
Consent to participate
All participants have obtained informed consent.
Human and animal rights statement and Informed consent
And this study has passed ethical review, all enrolled patients have agreed to participate and signed informed consent forms.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, FM., Xu, J., He, QY. et al. Association of serum interleukin-2 with severity and prognosis in hospitalized patients with community-acquired pneumonia: a prospective cohort study. Intern Emerg Med (2024). https://doi.org/10.1007/s11739-024-03699-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11739-024-03699-0