Abstract
Background
Metabolic dysfunction-associated steatotic liver disease (MASLD) represents the hepatic manifestation of increased adiposopathy, whose pathogenetic features have been proposed as tumourigenic triggers for colorectal cancer (CRC). We aim to identify specific metabolic signatures involved in CRC development that may be used as non-invasive biomarkers, paving the way for specific and personalized strategies of CRC prevention and early detection.
Methods
We retrospectively assessed CRC onset during a time frame of 8 years in a cohort of 1145 out-patients individuals who had previously been evaluated for Metabolic Syndrome.
Results
28 patients developed CRC. No association between CRC development and visceral and general obesity was detected, while baseline fasting plasma glucose (FPG) and non-invasive liver fibrosis scores were significantly higher in patients with CRC, compared to those who did not develop cancer. Liver steatosis and MASLD were more frequently diagnosed in patients who developed CRC compared to no cancer developers. Canonical correlations among metabolic biomarkers were not present in CRC developers, differently from no cancer group. In ROC analysis, FPG and non-invasive scores also showed good sensitivity and specificity in predicting colon cancer. We then calculated ORs for metabolic biomarkers, finding that higher FPG and non-invasive scores were associated with an increased risk of developing CRC.
Conclusion
MASLD and increased FPG may play a role in the clinical background of CRC, bringing to light the fascinating possibility of a reversed gut–liver axis communication in the pathogenesis of CRC. Thus, the use of non-invasive scores of fatty liver may be helpful to predict the risk of CRC and serve as novel prognostic factors for prevention and therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies in the Western world [1], ranking as the second leading cause of cancer-related death [2]. Although colonoscopy represents the gold standard for CRC screening and diagnosis, the identification of novel blood-based biomarkers for CRC prediction are welcome since they would be minimally invasive and easily accepted by patients. Indeed, one challenge clinicians face is to identify patients at high risk, as they would benefit from peculiar screening and follow-up [3]. Recently, a growing body of evidence highlighted how tumour onset and progression is a synergistic process in which different players give a substantial contribution, pointing at obesity as the strongest risk factor contributing to global cancer burden [4].
Also CRC may be considered an obesity-related cancer [5] and multiple mechanisms are believed to drive this association, including hyperinsulinemia, inflammation, and dyslipidemia [6] with a direct role of hyperinsulinemia, more strongly than other aspects of Metabolic Syndrome (MetS) [7]. Patients with hyperinsulinemia are susceptible to pro-inflammatory state, that has been shown to promote mutagenesis, tissue damage, and ultimately carcinogenesis [8], and pro-thrombotic state that promotes colon cancer growth through platelet hyperactivity [9]. Also the hepatic manifestation of the multisystem disorder linked to increased visceral adipose tissue, defined as Metabolic dysfunction-associated steatotic liver disease (MASLD), has been proposed as risk factor for CRC [10,11,12]. An umbrella review of meta-analysis and observational studies have shown a 50% increased incidence of CRC in patients with diabetes [13], and according to a recent systematic review evaluating 14 studies, the incidence of CRC was 1.43 per 1000 person-years in patients with MASLD [14] that may drive CRC at younger age, compared to subjects without fatty liver [14]. When investigating cancer prevalence in MASLD patients, CRC was among the most frequently reported, and MASLD was associated with an increase in cancer-related mortality [15]. The disease process in MASLD is best described by the grade of activity and the stage of liver fibrosis [16], rather than a dichotomous classification (steatosis vs. steatohepatitis), and patients with advanced fibrosis seems to have a more significant association to increased CRC risk [17]. Therefore, the assessment and staging of liver fibrosis may help identifying high-risk subgroup for CRC among people with hepatic steatosis. Yet, fibrosis can be assessed only through liver biopsy and elastography that, similarly to colonoscopy, are not feasible as wide-spread examinations, considering biopsy invasiveness, the high prevalence of MASLD, and the need for monitoring its progression. In this context, several easy-to-use non-invasive tests (NITs) have been proposed as bio-humoral and clinical surrogates and have been recommended by clinical guidelines in individuals with fatty liver and suspected advanced fibrosis to perform appropriate risk stratification and predict morbidity and mortality [18]. Furthermore, their values also showed a stronger association with specific types of cancer [19].
For these reasons, the goal of this study was to identify specific metabolic signatures involved in CRC development that could be used as non-invasive biomarkers, paving the way for specific and personalized strategies of CRC prevention and early detection in patients with fatty liver.
Materials and methods
Study design and patient involvement
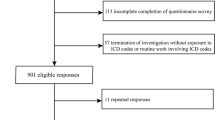
Between January and March 2023, we conducted telephonic interviews to retrospectively record the onset of CRC in 1266 individuals who had been previously evaluated for MetS at Internal Medicine Division “C. Frugoni” of University Hospital of Bari, Italy in the period between January 2015 and December 2020, excluding those with viral hepatitis and alcohol-related liver disease. When patients reported cancer diagnosis, we confirmed CRC by retrieving biopsy results directly from the hospital archival files, and when we could not find such data, we invited study participants to hand in their medical documentation.
They had been assigned a unique ID and registered in the electronic health register of Metabolic Diseases of the Department of Interdisciplinary Medicine at “Aldo Moro” University of Bari. After excluding those who had been diagnosed with other cancer types (n = 121), the final analysis was performed on a population of 1145 subjects. At the time of enrolment, this study (n.311, MSC/PBMC/2015) was approved by the Ethics Committee of the Azienda Ospedaliero-Universitaria Policlinico di Bari (Bari, Italy), in accordance with the requirements of the Declaration of Helsinki. Written informed consent for the use of clinical data was obtained from all participants in this study. In accordance with the approved Ethics Committee, only patients who were already 18 years old or more were included.
Data collection
At the time of enrolment, a standardized questionnaire collecting detailed information on reproductive history, smoking and alcohol drinking history, exposure to environmental toxics, medical history, educational level, and other socioeconomic and lifestyle variables was administered to patients (Supplementary Table 1). Information on drug use, occupation and family history of cancer had also been collected. Moreover, physical examination, anthropometric measures, biochemical assessment, and abdomen ultrasound to detect liver steatosis were performed. After an overnight fasting, patients underwent an abdominal ultrasound scanning performed by two expert physicians with more than 10 years of experience in ultrasonography with a 3.5–5 MHz convex probe (Esaote MyLab 70 Gold ultrasound system) [20].
Anthropometric assessment was performed using standardized procedures. Briefly, waist circumference (WC) was measured at the midpoint between the inferior part of the 12th costa and the anterior–superior iliac crest. Body Mass Index (BMI) was computed as weight (Kg) divided by the height squared (sqm) and BMI values (Kg/sqm) between 25 and 29.9 and over 30.0 were considered as overweight and obesity conditions, respectively. MetS was diagnosed according to International Diabetes Federation (IDF) definition [21] and visceral obesity was defined for WC values above 80 cm in women and 94 cm in men. Type 2 Diabetes (T2D) was diagnosed when glycosylated haemoglobin (HbA1c) ≥ 48 mmol/mol and/or fasting plasma glucose (FPG) ≥ 126 mg/dl and/or ongoing treatment for diabetes, while prediabetes was defined as impaired fasting glycaemia (100 < FPG < 126) and/or 42 < HbA1c < 48. MASLD diagnosis was based on the presence of liver steatosis identified by ultrasound and at least one of the five criteria for MetS, also considering BMI ≥ 25 kg/sqm to assess overweight or obesity alternatively to increased WC [22].
Non-invasive scores were determined according to published formulas (see Supplementary Table 2). Specifically, we calculated AST to ALT ratio (AAR), AST to Platelet Ratio Index (APRI); (AST to ALT ratio) to platelet ratio index (AARPRI), BMI, ALT, Age, and Triglycerides (BAAT); Fibrosis-4 Index (FIB-4); modified FIB-4 (mFIB-4), Fatty Liver Index (FLI), Hepatic Steatosis Index (HIS), NAFLD-Liver Fat Score (NAFLD-LFS), NAFLD Fibrosis Score (NFS), BARD score, Forns Score, and NFS-Ridge score.
Morning blood samples were obtained after 12 h of fasting from the antecubital veins, and then biochemical markers of glucose and lipid metabolism were measured in patients’ serum. After blood clotting and centrifugation, serum was processed, and liver and thyroid markers were measured following standardized biochemical procedures. All biochemical measurements were centralized and performed in the ISO 9001 certified laboratories of the University Hospital of Bari.
Data analysis
Descriptive statistical analyses of the study sample were performed, and results are expressed as mean ± standard deviation (SD) for numerical data, in counts and percentages for categorical data. Comparisons of clinical variables between two groups were conducted with Mann–Whitney U Test, while comparisons between categorical variables were performed with chi-squared test. To investigate the role of possible confounders, we performed analysis of covariance (ANCOVA) and computed the T-test for the difference between group means adjusted for the covariate. All reported p-values (p) were based on two-sided tests and compared to a significance level of 5%.
Empirical ROC curves were plotted along with a calculation of the area under the curve (AUC) to give us a measure of the capability to distinguish between CRC and no cancer groups and Youden’s Index (YI), or equivalently, the highest sensitivity + specificity, was used to determine the optimal cut-off.
Chi-square test, along with Fisher’s exact test if indicated, was used to study the association between categorical variables and Odds Ratio (OR) with their relative 95% confidence interval (95% CI) was calculated. Correlations among variables were also analysed and estimated using Spearman correlation coefficient (r). All analyses were performed using the NCSS 2023 Statistical Software (2023, NCSS, LLC. Kaysville, Utah, USA) and GraphPad Prism, version 10 (GraphPad Software; San Diego, CA, USA).
Results
Baseline characteristics of study population
Out of 1145 subjects (538 males, 607 females), 28 (2.45%; 22 males, 6 females) were diagnosed with CRC during the last eight years.
Mean age of population sample was 56.1 ± 15 years. WC (97.8 ± 14.7) was above the established cut-off for MetS diagnosis, as along with a mean BMI (27.3 ± 5.8) depicting a condition of overweight. When considering bio-humoral values, we found that mean FPG was 101.3 ± 29.9 and HbA1c was 41.6 ± 12.5 depicting an overall condition of prediabetes. Liver steatosis was US detected in 525 individuals and MASLD was diagnosed in 495 of them.
Table 1 summarizes all baseline characteristics of the population.
Biomarkers comparisons between CRC and control groups
We then performed a comparison between clinical and biochemical features of patients who developed CRC and did not (Table 2). Patients who developed CRC were older (p < 0.05) and presented baseline increased FPG (p < 0.005) and glycosylated haemoglobin (HbA1c) (p < 0.05) compared to patients who did not develop CRC. Liver transaminases AST (p < 0.01) and GGT (p < 0.05) were significantly increased in patients who developed CRC, while no significant differences were detected in ALT and Alkaline Phosphatase (ALP) levels as well as in lipid profile.
We compared the presence of US liver steatosis in the two groups, finding that its proportion was significantly greater (p < 0.05) in CRC developers (68%) compared to no cancer group (45%). When considering MASLD, this difference was even more pronounced (p < 0.01) since all CRC developers with liver steatosis had also MASLD, while this was not true in subjects who did not develop CRC (43%), thus suggesting that an association with CRC development could be found for fatty liver and MASLD.
In respect of the significant differences regarding glycaemic profile, we then considered T2D and impaired fasting glycaemia conditions, finding that nor the first neither the second showed a significant association with CRC development, although a trend could be identified. We finally investigated if another risk factor as smoking habits could interfere or having an adding weight in colorectal carcinogenesis. Also in this case, we could not identify a significant difference between the two groups.
Considering NITs, FIB-4 (1.1 ± 0.7 vs 1.8 ± 1, p < 0.0001), mFIB-4 (2.2 ± 1.5 vs 3.3 ± 2.3, p < 0.001), FORNS (5.3 ± 1.8 vs 6.3 ± 1.3, p < 0.005), APRI (0.3 ± 0.2 vs 0.5 ± 0.4, p < 0.05), AARPRI (0.6 ± 0.3 vs 0.8 ± 0.5, p < 0.05), NFS (− 0.4 ± 2.1 vs 0.8 ± 1.6, p < 0.01), BAAT (2.1 ± 0.7 vs 2.5 ± 0.6, p < 0.01), and BARD (1.7 ± 1.2 vs 2.3 ± 1, p < 0.01) were found significantly increased in CRC group (Fig. 1). Conversely, there were no significant differences between the two groups regarding AAR (0.8 ± 0.3 vs 1 ± 0.5, p = ns), FLI (48.1 ± 31.5 vs 58.9 ± 30.2, p = ns), NFS-Ridge (− 5.9 ± 5.3 vs − 4.5 ± 7.9, p = ns), and HSI (35.7 ± 6.5 vs 37 ± 4.3, p = ns).
Variables which were significantly increased in patients who later developed colorectal cancer (CRC). Comparisons were performed by Mann–Whitney test and p-value < 0.05 was considered significant. The box plots show the median (second quartile), first and third quartile, and whiskers represent minimum and maximum values. *p < 0.05; **p < 0.005; ***p < 0.0001. FPG Fasting plasma glucose, HbA1c glycosylated haemoglobin, FIB-4 Fibrosis-4 index, mFIB-4 modified FIB-4, APRI AST to Platelet Ratio Index, AARPRI (AST to ALT ratio) to Platelet Ratio Index, NFS NAFLD Fibrosis Score; BAAT BMI, ALT, Age, and Triglycerides
Considering the significant difference in age between the two groups, we then performed an age-adjusted comparison for NITs, finding that FIB-4, APRI, ARRPRI, and BAAT still significantly distinguished between CRC developers and not-developers (Table 3).
Different correlations among metabolic biomarkers in CRC and control groups
Considering the significant increase in their values, we then tried to deepen correlations among FPG and NITs in cancer and control groups. While in no cancer population, canonical correlations of FPG with non-invasive scores were detected, these correlations were not shown in CRC population. Also, when considering anthropometric parameters such as WC and BMI, we found that their well-known relationships with glycaemia and NITs were already lost at baseline in patients who were later diagnosed with CRC (Fig. 2).
Correlations of fasting plasma glucose, waist circumference and BMI with non-invasive scores of fatty liver. Heatmaps show Spearman correlations (r) among FPG, WC, BMI, and non-invasive scores of fatty liver in patients with (CRC) and without (NO) colorectal cancer and corresponding p-values (p). FPG Fasting Plasma Glucose, BMI Body Mass Index, WC Waist Circumference, FIB-4 Fibrosis-4 index, mFIB-4 modified FIB-4, APRI AST to Platelet Ratio Index, AARPRI (AST to ALT ratio) to Platelet Ratio Index, NFS NAFLD Fibrosis Score, BAAT BMI, ALT, Age, and Triglycerides, ns not significant. p < 0.05 were considered significant
Risk of developing CRC
With the aim of determining if the above-considered biomarkers could be associated ahead of time with CRC onset, we then performed ROC curves of those variables that had been found significantly different at baseline in the two groups (Fig. 3). FPG and all non-invasive scores, except APRI, showed a significant AUC. Specifically, FPG showed AUC = 0.66 (p < 0.005), with a cut-off value of 97 mg/dL, a sensitivity of 0.68 and a specificity of 0.60. When considering NITs, FIB-4 showed the highest AUC (0.74, p < 0.001) and YI (0.41, sensitivity 0.84, specificity 0.57) for a cut-off value of 1.
ROC curves for colorectal cancer developers detection. ROC curves for detection of patients who later developed colorectal cancer are shown for FPG (A), FIB-4 (B), mFIB-4 (C), FORNS (D), APRI (E), AARPRI (F), NFS (G), BAAT (H), BARD (I). In the table (J) empirical estimation of area under curve (AUC) with 95% confidence intervals and two-sided upper p-values for null hypothesis AUC = 0.5 (*p < 0.05; **p < 0.005; ***p < 0.001), cut-off values with related sensitivity and specificity levels, and Youden’s Index (YI) are reported. FPG Fasting Plasma Glucose, FIB-4 Fibrosis-4 index, mFIB-4 modified FIB-4, APRI AST to Platelet Ratio Index, AARPRI (AST to ALT ratio) to Platelet Ratio Index, NFS NAFLD Fibrosis Score, BAAT BMI, ALT, Age, and Triglycerides, YI Youden’s Index
To assess the risk of developing CRC when baseline FPG or NITs are increased, we then calculated OR (Fig. 4), finding that all non-invasive scores showed a significant association with CRC (p < 0.05). FIB-4, that had already shown the best YI in ROC analysis, showed the highest OR (6.1, 95% CI 2.2–16.5, p < 0.001). Regarding FPG, the risk of getting CRC for a subject with increased FPG at baseline was more than three times higher than a subject with glycaemia levels under 97 mg/dL (OR = 3.2, 95% CI 1.5–7.3; p < 0.01). Finally, we also calculated OR for BMI and WC to determine whether the international cut-offs for overweight and visceral obesity are consistent with an increased CRC risk, but no association was found.
Risk of developing colorectal cancer in subjects with increased metabolic and anthropometric variables. Forest plot represents Odds Ratios (OR) with their relative 95% confidence interval (CI). Chi-squared test, along with Fisher’s exact test if indicated, was used to study the association between CRC and increased FPG, non-invasive liver fibrosis scores and anthropometric measures. *p < 0.05; **p < 0.005; ***p < 0.001. FPG Fasting Plasma Glucose, WC Waist Circumference, BMI Body Mass Index, M males, F females, FIB-4 Fibrosis-4 index, mFIB-4 modified FIB-4, APRI AST to Platelet Ratio Index, AARPRI (AST to ALT ratio) to Platelet Ratio Index, NFS NAFLD Fibrosis Score, BAAT BMI, ALT, Age, and Triglycerides
Discussion
In this study, we investigated whether some non-invasive metabolic biomarkers are associated with the risk for CRC, as a consequence of the pathogenetic involvement of fatty liver infarction in its pathogenesis.
In individuals who later developed CRC, we found increased baseline fasting glycaemia and HbA1c, showing that subjects with higher FPG had more than three times the risk of developing CRC. Since mean values of such parameters depicted a condition of diabetes, also the hypothesis that chronic inflammation and hyperinsulinemia may act as direct carcinogenetic factors should be considered. However, the evidence that canonical relationship between FPG and fatty liver scores are lost in patients who later develop CRC suggests that hyperglycaemia and insulin resistance should not be considered as stand-alone triggers for CRC but in the context of the broad metabolic derangements associated to unhealthy lifestyle [23]. Since exacerbated fatty acid metabolism is an additional lipid signature of tumour cells [24], the derangement of canonical correlations among biochemical markers in our CRC group may mirror the uncoupling of cholesterol and lipid metabolism that are important drivers of tumour progression [25, 26]. Different microenvironmental factors, such as those inducing endoplasmic reticulum stress, promote fatty acid synthesis and dampen immune responses [27]. Similarly to hepatocytes, cancer cells increase the uptake of dietary free fatty acids and lipoproteins or upregulate the endogenous lipogenesis and cholesterol synthesis to answer the high lipid and cholesterol demands, necessary to sustain endlessly proliferating cells. These pathways contribute to invasion and metastasis through transcriptional and epigenetic changes, also promoting inflammation in the tumour microenvironment, and avoiding immune destruction [28]. Consequently, in patients with subsequent CRC development, the baseline levels of cholesterol and triglycerides could result normal because they are already fuelling cancer cells growth, as also supported by a previous study in which no associations between CRC risk and HDL-c and LDL-c levels were detected [29].
The not-significant association we found between obesity (assessed through both BMI and WC) and CRC development may apparently contrast with the evidence about adiposopathy role in cancer development and mortality [30, 31]. It is important to point out that values of WC registered in our cohort were barely above the IDF cut-offs for MetS and that mean BMI values depict a condition of overweight but not obesity in both no cancer and CRC group. Moreover, also the possible weight loss due to underdiagnosed CRC cases at baseline should be considered among reasons of such inconsistency.
On the other hand, we also propose another fascinating scenario to explain these apparently paradoxical findings. Since alterations of cholesterol metabolism influence tumour cells and increased cholesterol biosynthesis also promotes inflammation [32], this reprogramming of metabolic pathways could be not a consequence of cancer, but the reason for CRC development, eventually anticipating carcinogenesis. Indeed, NITs are markers of hepatocytes fat infarction that, acting as triglycerides depot, may explain why no differences were found between our two groups in circulating triglycerides levels, despite the increase of NITs in CRC developers.
In this context, higher incidence in economically developed countries compared to developing countries highlights the crucial role of diet in determining CRC onset [33] as the major modifiable risk factor [34, 35]. Among the pro- and antitumoural dietary factors, lipids appear to be of crucial importance since high-fat diet (HFD) consumption, especially with high saturated fatty acids (SFA) content, favours hyperproliferation of intestinal stem cells [36] while the supplementation with oleic acid, the predominant fatty acid component of olive oil, reduces intestinal inflammation and tumour development [37]. In the liver, HFD results in an altered saturated to monounsaturated fatty acid (MUFA) ratio, with the concomitant decreased de novo lipogenesis programmes and increased fatty acids β-oxidation pathways, through the suppression of stearoyl CoA desaturase 1 (SCD1) [38].
Furthermore, HFD also induces alterations in the microbial profile and bile acid metabolism [39] that encourage tumourigenesis. While diet shapes the gut microbiota, probably on the basis of selection of bacteria species as dictated by the quantity and quality of ingested nutrients [40], a vicious circle is established by which specific gut bacteria affect carbohydrate metabolism and are associated with insulin resistance [41] and metabolic changes that alter the cancer metabolome to create conditions conductive for normal to cancer cell transformation [42]. That is why we also considered MASLD, the liver manifestation of metabolic diseases and its non-invasive scores for the assessment of CRC risk, thus paving the way to propose fatty liver as the dominant common link driving CRC and obesity association.
This study presents some limitations that need to be discussed. First, since it is a retrospective study, it can only define an association but not a causal relationship between metabolic impairment and cancer development. Secondly, although NITs included in the study were created for different specific conditions (i.e. liver steatosis or fibrosis), we considered them as a whole mix to better encompass the wide clinical range of liver impairment that may occur in MASLD [43]. Finally, ROC analysis for the prediction of CRC showed AUC values which are not high enough to suggest a single test use in clinical practice. However, the aim of this study was to identify any condition that could highlight the need for further investigation and more frequent follow-up in at-risk patients and not to verify whether a single score could be useful as a standing-alone marker, since NITs should always be interpreted according to the clinical context and considering the results of other tests (biochemical, radiological, and endoscopic) [44]. Thus, we further underline that each NIT, along with fasting hyperglycaemia, should be considered in the context of the broad metabolic derangements associated to MASLD.
Here, we postulate a new paradigm pointing at the liver–gut axis in which hepatocytes inflammation due to fat infarction together with increased fasting glucose levels represent the primum movens of metabolic derangements leading to CRC development. Due to the intertwined relationship between the gut and liver, the gut may represent the first target of systemic metabolic impairment due to fatty liver disease through the action of several enterokines and hormones. Owing to our findings, it is crucial to properly diagnose MASLD to offer patients a more tailored follow-up and screening programmes to minimize the risk of CRC development and to strengthen the importance of healthy behaviours in cancer prevention.
References
Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A (2017) Colorectal cancer incidence patterns in the United States, 1974–2013. JNCI J Natl Cancer Inst. https://doi.org/10.1093/jnci/djw322
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, Fournier C, Staufer K, Stauber RE, Bugianesi E et al (2022) Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 71:1006–1019. https://doi.org/10.1136/gutjnl-2021-324243
GBD (2019) Cancer risk factors collaborators the global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the global burden of disease study 2019. Lancet Lond Engl 2022(400):563–591. https://doi.org/10.1016/S0140-6736(22)01438-6
Abar L, Vieira AR, Aune D, Sobiecki JG, Vingeliene S, Polemiti E, Stevens C, Greenwood DC, Chan DSM, Schlesinger S et al (2018) Height and body fatness and colorectal cancer risk: an update of the WCRF-AICR systematic review of published prospective studies. Eur J Nutr 57:1701–1720. https://doi.org/10.1007/s00394-017-1557-1
Crudele L, Piccinin E, Moschetta A (2021) Visceral adiposity and cancer: role in pathogenesis and prognosis. Nutrients 13:2101. https://doi.org/10.3390/nu13062101
Liu JJ, Druta M, Shibata D, Coppola D, Boler I, Elahi A, Reich RR, Siegel E, Extermann M (2014) Metabolic syndrome and colorectal cancer: is hyperinsulinemia/insulin receptor-mediated angiogenesis a critical process? J Geriatr Oncol 5:40–48. https://doi.org/10.1016/j.jgo.2013.11.004
Kay J, Thadhani E, Samson L, Engelward B (2019) Inflammation-induced DNA damage. Mutations Cancer DNA Repair 83:102673. https://doi.org/10.1016/j.dnarep.2019.102673
Cariello M, Piccinin E, Zerlotin R, Piglionica M, Peres C, Divella C, Signorile A, Villani G, Ingravallo G, Sabbà C et al (2021) Adhesion of platelets to colon cancer cells is necessary to promote tumor development in xenograft. Genet Inflamm Models Cancers 13:4243. https://doi.org/10.3390/cancers13164243
Lee H, Lee HW, Kim SU, Chang Kim H (2022) Metabolic dysfunction-associated fatty liver disease increases colon cancer risk: a nationwide cohort study. Clin Transl Gastroenterol 13:e00435. https://doi.org/10.14309/ctg.0000000000000435
Mantovani A, Dauriz M, Byrne CD, Lonardo A, Zoppini G, Bonora E, Targher G (2018) Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: a systematic review and meta-analysis. Metabolism 87:1–12. https://doi.org/10.1016/j.metabol.2018.06.004
Chen J, Bian D, Zang S, Yang Z, Tian G, Luo Y, Yang J, Xu B, Shi J (2019) The association between nonalcoholic fatty liver disease and risk of colorectal adenoma and cancer incident and recurrence: a meta-analysis of observational studies. Expert Rev Gastroenterol Hepatol 13:385–395. https://doi.org/10.1080/17474124.2019.1580143
Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350:g7607. https://doi.org/10.1136/bmj.g7607
Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM (2019) The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—a longitudinal cohort study. J Hepatol 71:1229–1236. https://doi.org/10.1016/j.jhep.2019.08.018
George ES, Sood S, Kiss N, Daly RM, Nicoll AJ, Roberts SK, Baguley BJ (2022) The evidence surrounding non-alcoholic fatty liver disease in individuals with cancer: a systematic literature review. Curr Oncol 30:48–74. https://doi.org/10.3390/curroncol30010005
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour J-F, Schattenberg JM et al (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73:202–209. https://doi.org/10.1016/j.jhep.2020.03.039
Kim MC, Park JG, Jang BI, Lee HJ, Lee WK (2019) Liver fibrosis is associated with risk for colorectal adenoma in patients with nonalcoholic fatty liver disease. Medicine (Baltimore) 98:e14139. https://doi.org/10.1097/MD.0000000000014139
Lazarus JV, Castera L, Mark HE, Allen AM, Adams LA, Anstee QM, Arrese M, Alqahtani SA, Bugianesi E, Colombo M et al (2023) Real-world evidence on non-invasive tests and associated cut-offs used to assess fibrosis in routine clinical practice. JHEP Rep Innov Hepatol 5:100596. https://doi.org/10.1016/j.jhepr.2022.100596
Kim G-A, Lee HC, Choe J, Kim M-J, Lee MJ, Chang H-S, Bae IY, Kim H-K, An J, Shim JH et al (2018) Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 68:140–146. https://doi.org/10.1016/j.jhep.2017.09.012
Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L, Repetto G (2004) The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg 14:635–637. https://doi.org/10.1381/096089204323093408
Alberti KGMM, Zimmet P, Shaw J (2006) Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med J Br Diabet Assoc 23:469–480. https://doi.org/10.1111/j.1464-5491.2006.01858.x
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP et al (2023) A multi-society delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. https://doi.org/10.1016/j.jhep.2023.06.003
Bays HE, González-Campoy JM, Henry RR, Bergman DA, Kitabchi AE, Schorr AB, Rodbard HW (2008) Adiposopathy working group is adiposopathy (sick fat) an endocrine disease? Int J Clin Pract 62:1474–1483. https://doi.org/10.1111/j.1742-1241.2008.01848.x
Phan AT, Goldrath AW, Glass CK (2017) Metabolic and epigenetic coordination of T cell and macrophage immunity. Immunity 46:714–729. https://doi.org/10.1016/j.immuni.2017.04.016
Bovenga F, Sabbà C, Moschetta A (2015) Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab 21:517–526. https://doi.org/10.1016/j.cmet.2015.03.002
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777. https://doi.org/10.1038/nrc2222
Raccosta L, Fontana R, Corna G, Maggioni D, Moresco M, Russo V (2016) Cholesterol metabolites and tumor microenvironment: the road towards clinical translation. Cancer Immunol Immunother CII 65:111–117. https://doi.org/10.1007/s00262-015-1779-0
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho H-I, Celis E, Lennox B et al (2010) Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 16:880–886. https://doi.org/10.1038/nm.2172
Borgquist S, Butt T, Almgren P, Shiffman D, Stocks T, Orho-Melander M, Manjer J, Melander O (2016) Apolipoproteins, lipids and risk of cancer: apolipoproteins, lipids and risk of cancer. Int J Cancer 138:2648–2656. https://doi.org/10.1002/ijc.30013
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet Lond Engl 371:569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638. https://doi.org/10.1056/NEJMoa021423
Taniguchi K, Karin M (2018) NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 18:309–324. https://doi.org/10.1038/nri.2017.142
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 25:16–27. https://doi.org/10.1158/1055-9965.EPI-15-0578
Farinetti A, Zurlo V, Manenti A, Coppi F, Mattioli AV (2017) Mediterranean diet and colorectal cancer: a systematic review. Nutrition 43–44:83–88. https://doi.org/10.1016/j.nut.2017.06.008
Veettil SK, Wong TY, Loo YS, Playdon MC, Lai NM, Giovannucci EL, Chaiyakunapruk N (2021) Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Netw Open 4:e2037341. https://doi.org/10.1001/jamanetworkopen.2020.37341
Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong S-J, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E et al (2016) High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531:53–58. https://doi.org/10.1038/nature17173
Ducheix S, Peres C, Härdfeldt J, Frau C, Mocciaro G, Piccinin E, Lobaccaro J-M, De Santis S, Chieppa M, Bertrand-Michel J et al (2018) Deletion of stearoyl-CoA desaturase-1 from the intestinal epithelium promotes inflammation and tumorigenesis, reversed by dietary oleate. Gastroenterology 155:1524-1538.e9. https://doi.org/10.1053/j.gastro.2018.07.032
Piccinin E, Cariello M, De Santis S, Ducheix S, Sabbà C, Ntambi JM, Moschetta A (2019) Role of Oleic acid in the gut-liver axis: from diet to the regulation of its synthesis via stearoyl-CoA desaturase 1 (SCD1). Nutrients 11:2283. https://doi.org/10.3390/nu11102283
Gadaleta RM, Cariello M, Crudele L, Moschetta A (2022) Bile salt hydrolase-competent probiotics in the management of IBD: unlocking the “bile acid code.” Nutrients 14:3212. https://doi.org/10.3390/nu14153212
Petruzzelli M, Moschetta A (2010) Intestinal ecology in the metabolic syndrome. Cell Metab 11:345–346. https://doi.org/10.1016/j.cmet.2010.04.012
Takeuchi T, Kubota T, Nakanishi Y, Tsugawa H, Suda W, Kwon AT-J, Yazaki J, Ikeda K, Nemoto S, Mochizuki Y et al (2023) Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 621:389–395. https://doi.org/10.1038/s41586-023-06466-x
Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, Ivanisevic J, Cho K, Wick EC, Hechenbleikner EM et al (2015) Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab 21:891–897. https://doi.org/10.1016/j.cmet.2015.04.011
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402, https://doi.org/10.1016/j.jhep.2015.11.004.
EASL-ALEH Clinical Practice Guidelines (2015) Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 63:237–264. https://doi.org/10.1016/j.jhep.2015.04.006
Acknowledgements
The authors thank the physicians and nurses of the Unità Operativa Complessa Universitaria di Medicina Interna “Cesare Frugoni” of the Azienda Ospedaliero—Universitaria Policlinico di Bari for their help and support during the study. They would like to pay special thanks to Roberta Le Donne for her support.
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement. A.M. is funded by AIRC IG 2019 “Regulation of lipid metabolic pathways in the gut–liver axis: relevance in hepatocarcinoma”. Id. 23239; MIUR- PRIN Progetti di Ricerca di Rilevante Interesse Nazionale 2022. “Metabolic hits in the road to colon cancer”. Codice progetto n. 2022H9MPZ5; Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2 Investment 1.4—Call for tender No. 3138 of 16/12/2021 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code: CN00000041, CUP H93C22000430007, Project title “National Center for Gene Therapy and Drugs based on RNA Technology”. Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2 Investment 1.3—Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods”. Project funded by the European Union—Next Generation EU—PNRR M6C2—Investimento 2.1 Valorizzazione e potenziamento della ricerca biomedica del SSN" Project code PNRR-MR1-2022–12376395. CUP H93C22000780006. Project title: Italian Autoimmune Liver Disease (IT-AILD) Clinical Research Network (CRN). L.C. is funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Award Number: Project code PE0000015, Concession Decree No. 1243 of 2 August 2022 adopted by the Italian Ministry of University and Research, CUP H33C22000680006, Project title “Ageing well in an ageing society—A novel public–private alliance to generate socioeconomic, biomedical and technological solutions for an inclusive Italian ageing society– AGE-IT". E.P. is funded by PON-AIM1853334, Attività 2- Linea 1. R.M.G. is funded by PON “RICERCA E INNOVAZIONE” 2014–2020—Innovazione (D.M. 10 AGOSTO 2021, N. 1062). The funders had no role in study design, investigation, interpretation, or writing of the paper.
Author information
Authors and Affiliations
Contributions
L.C. and A.M. contributed to conceptualization; L.C. and G.G. contributed to methodology and visualization; L.C., C.D.M., and G.G. provided software and did formal analysis; L.C., F.N., S.P., E.D.B., and A.M. performed investigation; L.C. and C.D.M. performed data curation; L.C. was involved in writing—original draft preparation; E.P. and M.C. were involved in writing—review and editing; R.M.G. and A.M. did supervision; A.M. performed project administration; L.C., R.M.G., E.P., and A.M. contributed to funding acquisition. All authors have read and agreed to submit the current version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Data availability
Data presented in this study are available on request from the corresponding author.
Informed consent
All participants provided informed consent.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crudele, L., De Matteis, C., Novielli, F. et al. Fasting hyperglycaemia and fatty liver drive colorectal cancer: a retrospective analysis in 1145 patients. Intern Emerg Med (2024). https://doi.org/10.1007/s11739-024-03596-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11739-024-03596-6