Abstract
Heart failure (HF) is associated with poor outcome after stroke, but data from large prospective trials are sparse.
We assessed the impact of HF on clinical endpoints in patients hospitalized with acute ischemic stroke or transient ischemic attack (TIA) enrolled in the prospective, multicenter Systematic Monitoring for Detection of Atrial Fibrillation in Patients with Acute Ischemic Stroke (MonDAFIS) trial. HF was defined as left ventricular ejection fraction (LVEF) < 55% or a history of HF on admission. The composite of recurrent stroke, major bleeding, myocardial infarction, and all-cause death, and its components during the subsequent 24 months were assessed. We used estimated hazard ratios in confounder-adjusted models. Overall, 410/2562 (16.0%) stroke patients fulfilled the HF criteria (i.e. 381 [14.9%] with LVEF < 55% and 29 [1.9%] based on medical history). Patients with HF had more often diabetes, coronary and peripheral arterial disease and presented with more severe strokes on admission. HF at baseline correlated with myocardial infarction (HR 2.21; 95% CI 1.02–4.79), and all-cause death (HR 1.67; 95% CI 1.12–2.50), but not with major bleed (HR 1.93; 95% CI 0.73–5.06) or recurrent stroke/TIA (HR 1.08; 95% CI 0.75–1.57). The data were adjusted for age, stroke severity, cardiovascular risk factors, and randomization. Patients with ischemic stroke or TIA and comorbid HF have a higher risk of myocardial infarction and death compared with non-HF patients whereas the risk of recurrent stroke or major hemorrhage was similar. Trial registration number Clinicaltrials.gov NCT02204267.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of both stroke and heart failure (HF) is high in the elderly, and there is a long list of common cardiovascular risk factors, including hypertension, diabetes, sleep apnea, kidney dysfunction, or atrial fibrillation (AF). HF is regarded as an independent risk factor for ischemic stroke, and about 9% of all ischemic strokes are assumed to be related to HF [1]. Furthermore, there is an association between HF with unfavorable clinical outcome and mortality after stroke [2,3,4,5,6]. In addition, a reduced ejection fraction in patients with ischemic stroke and atrial fibrillation is associated with mortality [7]. In contrast, it is less clear whether HF is also associated with recurrent vascular events following an ischemic stroke. Data on recurrent ischemic stroke in stroke patients with HF are conflicting: a systemic review and meta-analysis of seven prospective trials with differing follow-up times demonstrated a significant association of HF with recurrent ischemic stroke in 9173 ischemic stroke patients [8]. However, a retrospective insurance data-based analysis showed no significant association of recurrent stroke and HF in 370,527 ischemic stroke patients [9]. While there are several publications focusing on myocardial infarction following ischemic stroke [10, 11], data on the association of myocardial infarction with HF in ischemic stroke patients are scarce [12]. In addition, studies examining the occurrence of major bleeding in association with heart failure in ischemic stroke patients are missing.
In this post-hoc analysis of the prospective multicenter MonDAFIS study, we analyzed the impact of HF at baseline on the composite of recurrent stroke, major bleeding, myocardial infarction, and all-cause death within 24 months after hospitalization for acute ischemic stroke or transient ischemic attack (TIA) [13, 14]. Furthermore, we investigated the association of each vascular endpoint separately with HF and rates of oral anticoagulation after 24 months in patients with or without HF at baseline.
Methods
Study cohort
MonDAFIS was an investigator-initiated randomized trial sponsored by the Charité—Universitätsmedizin Berlin, Berlin, Germany, and supported by an unrestricted research grant from Bayer Vital GmbH, Leverkusen, Germany to the Charité. The study rationale and design [12] as well as the primary and secondary endpoints [14] were published previously. The MonDAFIS study received primary approval from the Ethics Committee of the Charité—Universitätsmedizin Berlin, Germany. All 39 participating study centers provided approval from their respective ethics committees. All study patients gave written informed consent. The MonDAFIS trial complies with the Declaration of Helsinki. Patients were recruited from December, 2014 to September, 2017. A critical event committee adjudicated all serious adverse events (including recurrent stroke, myocardial infarction, major bleeding, and all-cause death). The members met at the study coordinating center and were blinded to trial randomization.
Study population
Men or women ≥ 18 years of age were eligible for study enrollment if they had an ischemic stroke according to WHO criteria [15]. If the newly occurring neurological deficits were transient in nature (consistent with a TIA), an additional inclusion criterion required either a neurologist to have observed and documented the acute neurological deficits or evidence of a corresponding acute ischemic lesion on imaging. Patients were excluded due to withdrawal of informed consent and data deletion or lack of any data. Detailed information regarding further in- and exclusion criteria as well as the trial intervention was published previously [14].
In the vast majority of MonDAFIS patients, left ventricular ejection fraction (LVEF) was determined by echocardiography at baseline as part of routine diagnostic procedures. The LVEF was captured in the eCRF as a categorized variable (LVEF ≥ 55% (“normal”), LVEF 31–54% (“slightly reduced”), LVEF ≤ 30% (“reduced”)). The rationale for this definition was based on the ESC guidelines for measuring LVEF that was valid at the time of study conception. In addition, heart failure (HF) was considered as pre-existing at baseline if it was reported by the patient or evident from existing medical reports (history of HF). In the present analysis, patients with a documented LVEF < 55% and/or a history of HF at baseline were considered HF patients. Patients without echocardiography during the index-stroke/TIA related hospital stay were excluded from the present analysis.
Outcomes
In line with the pre-defined secondary endpoint of the MonDAFIS study, we assessed the composite of recurrent stroke, myocardial infarction, major bleed, and all-cause death within 2 years after the index stroke in defined subgroups. Furthermore, the individual components of the composite endpoint were analyzed separately. The rate of oral anticoagulation within 24 months after the index event was assessed in patients with and without HF at baseline.
Statistical methods
This is an explorative analysis of the MonDAFIS study data set using predefined outcomes. Baseline characteristics are reported as frequencies and percentages for categorical variables or median and limits of the interquartile range (IQR; [25th and 75th percentile]) for metric variables. For the outcomes of interest, we conducted event-free survival analyses comparing cumulative hazards between patient groups. Event-free survival time, as well as survival, was measured in person-days until one of the events of the combined endpoint or death occurred, the study ended, or the participant was lost to follow-up. These dropouts are censored at the time of last contact. We used Kaplan–Meier curves and the log-rank test to compare crude cumulative hazard distributions. Cox Proportional Hazard models (crude and adjusted for age and cardivascular risk factors as arterial hypertension, diabetes mellitus, hyperlipoproteinemia, coronary heart disease, detection of atrial fibrillation and group randomization as well as stroke severity measured by the NIHSS score at baseline given as model 1 and model 2 with further adjustment for intravenous thrombolysis and endovascular thrombectomy in addition) were used to estimate hazard ratios (HR) for each vascular endpoint, the composite endpoint for all-cause death risk within 2 years after ischemic stroke or TIA. In a further sensitivity analysis, we added the status of antithrombotic therapy (antiplatelets or oral anticoagulation) at discharge after the index stroke in to the above-mentioned models. Cox Proportional Hazard assumption was checked. For the endpoint ‘oral anticoagulation 24 months after index event’ a multiple binary logistic regression analysis (adjusted for AF and detection of left atrial and/or ventricular thrombus) was conducted and adjusted odds ratios, as well as respective confidence intervals (CI) are reported. Data preparation was done using the software IBM SPSS Statistics 24.
Results
Of 3,431 patients included in MonDAFIS, LVEF was available in 2562 (75%) patients (mean age 67 [57–76] years, 39.5% female, median NIHSS score on admission 2 points [1–4]). Figure 1 shows the derivation of the study population. Patients without documented LVEF were older, more often had a TIA as index event and a stroke or TIA before the index event (Table 1 Online Supplement).
Overall, 381/2562 (14.9%) patients had a LVEF < 55% and 75/2562 patients had a documented past medical history of HF (of which 29 had a LVEF ≥ 55%). Therefore, a total of 410/2562 (16.0%) patients fulfilled at least one of the pre-defined HF criteria (Fig. 1). Baseline characteristics and results of multiple binary logistic regression of patients with vs without HF are shown in Table 1.
Patients with HF more often had diabetes mellitus [OR 1.33; 95% CI 1.04–1.70; P = 0.026], coronary artery disease [OR 3.12; 95% CI 2.36–4.13; P < 0.001], and peripheral arterial disease [OR 2.03; 95% CI 1.27–3.24; P = 0.003] than non-HF patients. Moreover, patients with HF had a higher stroke severity on admission than patients without HF [assessed by the NIHSS score; OR per point 1.05; 95% CI 1.02–1.09; P = 0.002).
Association of heart failure with clinical outcomes
Within two years after index stroke or TIA, the predefined composite endpoint occurred in 480/2561 (14.0%) of patients, including recurrent stroke in 301 (8.8%) patients, myocardial infarction in 43 (1.3%) patients, major bleeding in 32 (0.9%) patients and all-cause death in 175 (5.1%) patients. Recurrent ischemic stroke or TIA occurred in 283/2561 (8.3%) patients, while 18/2561 (0.7%) patients had a hemorrhagic stroke within 2 years.
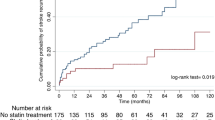
After adjusting for cardiovascular risk factors, stroke severity and randomization (model 1), HF was associated with myocardial infarction [HR 2.21; 95% CI 1.02–4.79; P = 0.046], and all-cause death [HR 1.67; 95% CI 1.12–2.49, P = 0.013] within 2 years after the index event. The event rates at different time frames for myocardial infarction and all-cause death are shown in Tables 2 and 3 Online Supplement. On the contrary, neither the composite endpoint [HR 1.28; 95% CI 0.99–1.66] nor recurrent ischemic stroke or TIA [HR 1.07; 95% CI: 0.74–1.55] nor major bleeding [HR 1.93; 95% CI 0.73–5.06] (Fig. 2 and Table 2) was significantly increased in stroke patients with HF. Additional adjustment for intravenous thrombolysis and endovascular thrombectomy (model 2) yielded similar results (Table 2). Also, excluding patients with a documented medical history of HF but normal LVEF (≥ 55%) at baseline yielded similar results (Table 4 Online Supplement). Adding the status of 'no-antithrombotic’ treatment at hospital discharge after the index stroke into both models of the cox regression analysis did not change the significant impact of heart failure in the occurrence of myocardial infarction or all-cause death.
Secondary stroke prevention in patients with heart failure
Information on oral anticoagulation at 24 months was available in 2072/2561 (80.9%) patients and, similarly, on antiplatelet treatment in 2075/2561 (81.0%) patients. Overall, 312/2,072 (15.1%) patients were on anticoagulation and 1,645/2,075 (79.4%) patients on antiplatelets at 24 months. In patients with HF at baseline, information on oral anticoagulation at 24 months was available in 304/410 (74.2%) patients. There was a statistically significant difference in the rate oral anticoagulation in HF patients vs. non-HF patients (63/304 [20.7%] vs. 249/1768 [14.1%], respectively, P = 0.004). AF was diagnosed in 46/63 (73.0%) of those with oral anticoagulants and HF. The rate of oral anticoagulation was 15.7% in HF patients vs 11.8% in non-HF patients at 6 months after the index stroke/TIA (p = 0.046) and 16.6% vs 12.9% after 12 months (P = 0.072) (Table 5 online supplement).
After multivariate adjustment (including newly detected AF within 24 months and left ventricular or left atrial thrombus at baseline), there was no significant association between HF status at baseline and oral anticoagulation after 24 months [OR 0.89; 95% CI 0.51–1.56; P = 0.689].
Discussion
The prospective multicenter MonDAFIS study shows that ischemic stroke or TIA patients with comorbid HF had a significantly higher rate of myocardial infarction and all-cause mortality within 24 months than non-HF patients. One strength of our post hoc analysis is the large number of clinical endpoints assessed by an independent endpoint committee and standardized follow-up for 24 months. Our data confirm that stroke/TIA patients with comorbid HF are not only more likely to have cardiovascular risk factors (such as diabetes, coronary heart disease, peripheral arterial disease) but also to have more severe strokes than stroke patients without HF [2]. Our finding of a statistically higher rate of myocardial infarction over 24 months after stroke/TIA in the presence of HF is consistent with the results of a large multicenter registry in the UK based on NHS primary care data of 9,840 patients [12]. The detected higher mortality rate in ischemic stroke patients with comorbid HF is also in line with previous studies [6, 9]. Of note, the rate of recurrent stroke as well as recurrent ischemic stroke or TIA was not associated with HF in our study, which was also found in a retrospective analysis of a large registry but is not consistent with a meta-analysis of prospective studies [8, 9]. The follow-up period of 2 years was may be too short to observe any potential association between HF and ischemic stroke—the authors of the WARCEF trial reported a higher rate of ischemic strokes during a follow-up period that was approximately 1.5 years longer. One study reported a significant association of recurrent intracerebral hemorrhage in patients with HF compared to non-HF patients [9]. In MonDAFIS, the HF status at baseline was not associated with the rate of major bleeding after stroke, even though 21% of HF patients received oral anticoagulation at 24 months. In fact, OAC status was not statistically different between HF vs. non-HF patients after adjusting for AF and presence of cardiac thrombi.
There are some limitations that should be mentioned. First, the MonDAFIS study was not designed to investigate the impact of HF on recurrent vascular events or death. Second, as echocardiography was conducted according to routine clinical practice respective data were available for only 75% of the total study population. Third, we had no systematic information on biomarkers, like natriuretic peptides. Fourth, we had no further information on HF status after hospital discharge after the index-stroke/TIA. Fifth, our results cannot be applied to all stroke patients, as patients had to provide informed consent to participate in the MonDAFIS study. Finally, we are not able to specify the cause of death. Due to formal data protection laws we did not have access to death certificates and autopsy is not regularly performed even if patients die in hospital.
Conclusion
Patients with acute ischemic stroke or TIA with comorbid HF have a higher prevalence of cardiovascular risk factors, suffered more severe (index) strokes and have a higher risk for myocardial infarctions or all-cause death within 24 months after the index stroke. Special attention should be paid to the prevention of myocardial infarction in this patient population and cardiac follow-up should be recommended in stroke patients with HF.
Data availability
Due to data protection regulations, we cannot release the data, as the patient consent form specifically defined or limited data sharing to the evaluating biometric center. Therefore, upon request, it can be decided whether a transfer to third parties can be facilitated through an ethics approval and data protection approval from the Charité.
References
Doehner W, Ural D, Haeusler KG et al (2018) Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 20(2):199–215
Barkhudaryan A, Doehner W, Scherbakov N (2021) Ischemic stroke and heart failure: facts and numbers. An update. J Clin Med 10(5):1146
Haeusler KG, Laufs U, Endres M (2011) Chronic heart failure and ischemic stroke. Stroke 42(10):2977–2982
Tai YH, Chang CC, Yeh CC et al (2020) Long-term risk of stroke and poststroke outcomes in patients with heart failure: two nationwide studies. Clin Epidemiol 12:1235–1244
Sennfalt S, Pihlsgard M, Petersson J, Norrving B, Ullberg T (2020) Long-term outcome after ischemic stroke in relation to comorbidity—an observational study from the Swedish Stroke Register (Riksstroke). Eur Stroke J 5(1):36–46
Scherbakov N, Haeusler KG, Doehner W (2015) Ischemic stroke and heart failure: facts and numbers. ESC Heart Fail 2(1):1–4
RAF and RENO-EXTEND Investigators (2023) The risk of stroke recurrence in patients with atrial fibrillation and reduced ejection fraction. Eur Stroke J 8(3):731–737
Katsanos AH, Parissis J, Frogoudaki A et al (2016) Heart failure and the risk of ischemic stroke recurrence: a systematic review and meta-analysis. J Neurol Sci 362:182–187
Pana TA, Wood AD, Perdomo-Lampignano JA et al (2019) Impact of heart failure on stroke mortality and recurrence. Heart Asia 11(1):e011139
Alqahtani F, Aljohani S, Tarabishy A, Busu T, Adcock A, Alkhouli M (2017) Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke 48(11):2931–2938
Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M (2018) Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol 17(12):1109–1120
Pana TA, Wood AD, Mamas MA et al (2019) Myocardial infarction after acute ischaemic stroke: Incidence, mortality and risk factors. Acta Neurol Scand 140(3):219–228
Haeusler KG, Kirchhof P, Heuschmann PU et al (2016) Impact of standardized MONitoring for Detection of Atrial Fibrillation in Ischemic Stroke (MonDAFIS): rationale and design of a prospective randomized multicenter study. Am Heart J 172:19–25
Haeusler KG, Kirchhof P, Kunze C et al (2021) Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): a randomised, open-label, multicentre study. Lancet Neurol 20(6):426–436
Hatano S (1976) Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 54(5):541–553
Funding
Open Access funding enabled and organized by Projekt DEAL. The MonDAFIS study was an investigator-initiated, prospective, randomized, multicenter study sponsored by the Charité—Universitätsmedizin Berlin, Germany and supported by an unrestricted research grant from Bayer Vital GmbH, Leverkusen, Germany to the Charité – Universitätsmedizin Berlin, Germany.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
PK is listed as inventor on two patents held by the University of Birmingham, Birmingham, UK (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). PK receives research support for basic, translational, and clinical research projects from the EU, British Heart Foundation, Leducq Foundation, the UK Medical Research Council, and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past. GT has received speakers' honoraria or consulting fees from Amarin, Daichi Sanyo, Acandis, Bayer Healthcare, Boehringer Ingelheim, Covidien, Bristol-Myers Squibb, Portola, Stryker, and Pfizer. DGN has received speakers' honoraria and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Novartis, and Pfizer. JR has received speakers' honoraria and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and AstraZeneca. UL reports honoraria and reimbursements for lectures, participation in studies, scientific cooperations (with Saarland University, Saarland, Germany), consulting, travel, support (of colleagues), or support of scientific meetings by Amgen, Bayer, Boehringer Ingelheim, Daiichi Sankyo, MSD, Sanofi, and Servier, outside the submitted work. RV reports grants, personal fees, and being a shareholder from Bayer; grants from Boehringer; grants and personal fees from Bristol-Myers Squibb and Pfizer; grants from Daiichi Sankyo, Medtronic, and Biogen; personal fees from Javelin, Abbott, and AstraZeneca; and holding shares in Novartis, outside the submitted work. RV is an investigator of Imperial National Institutes of Health Research Biomedical Research Centre and partially funded by the EU's Horizon 2020 research and innovation programme (grant agreement 754517 [PRESTIGE-AF]). PUH reports grants from Charité, Universitätsmedizin Berlin during study conduct (within MonDAFIS for biometry; member of the scientific board); research grants from the German Ministry of Research and Education, German Research Foundation, the Bavarian State (ministry for science and the arts; within STAAB COVID-19), the EU, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, Federal Joint Committee within the Innovationfond, University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi Sankyo), and University Göttingen (within FIND-AF(randomised); supported by an unrestricted research grant to the University Göttingen from Boehringer Ingelheim), outside the submitted work. KGH reports speakers' honoraria, consulting fees or lecture honoraria from Abbott, Alexion, AMARIN, AstraZeneca, Bayer Healthcare, Sanofi, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Bristol-Myers Squibb, Biotronik, Medtronic, Portola, Premier Research, SUN Pharma, WL Gore and Associates, and Edwards Lifesciences, as well as study support by Bayer and Getemed. ME reports grants from Bayer and fees paid to the Charité from AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Daiichi Sankyo, GlaxoSmithKline, Novartis, Pfizer, and Sanofi. All other authors declare no competing interests outside the submitted work.
Ethical Statements such as Human and animal rights statement and Informed consent
Each individual patient provided written consent for study inclusion and the associated collection of disease-related data. Furthermore, the study protocol was reviewed and positively evaluated by the Ethics Committee of the Charité and also by the individual ethics committees of the investigational centers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tütüncü, S., Olma, M.C., Kunze, C. et al. Heart failure, recurrent vascular events and death in patients with ischemic stroke—results of the MonDAFIS study. Intern Emerg Med (2024). https://doi.org/10.1007/s11739-024-03594-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11739-024-03594-8