Abstract
Abdominal pain in patients with diverticular disease (DD) can be challenging in clinical practice. Patients with symptomatic uncomplicated diverticular disease (SUDD) and patients with a previous acute diverticulitis (PD) may share a similar clinical pattern, difficult to differentiate from irritable bowel syndrome (IBS). We used standardized questionnaires for DD (short and long lasting abdominal pain) and IBS (following Rome III Criteria) to assess clinical features of abdominal pain, in terms of presence, severity and length, in SUDD and PD patients. One hundred and forty-eight SUDD and 118 PD patients completed all questionnaires. Short-lasting pain was more frequent in SUDD than PD patients (p = 0.007). Number of long-lasting pain episodes was higher in SUDD (6.6 ± 11.9) compared to PD patients (3.4 ± 6.9) (p < 0.001). PD patients reported long-lasting pain more frequently in the lower left abdomen (p < 0.001), while in SUDD it was more frequently diffuse (p = 0.002) or localized in the lower right quadrant (p = 0.009). Features associated with long-lasting pain (fever, confinement to bed, consultations, antibiotic therapy, hospitalization) were more often reported in PD patients. IBS criteria were reported in 28.2% of patients and were more frequent in SUDD than PD patients (37.2% vs 17.1%, p < 0.001). SUDD and PD patients presented different pattern of abdominal pain (length, number of long lasting episodes, site and associated features), with a third reporting overlap with IBS. Further observational studies are needed to better characterize abdominal symptoms in DD patients, especially in those not fulfilling IBS criteria.
Trial registration: The REMAD Registry is registered as an observational study in ClinicalTrial.gov (ID: NCT03325829).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colonic diverticula are a frequent condition in Western countries, which prevalence increases with age, affecting up to two-thirds of people older than 80 years [1]. Most patients will remain asymptomatic throughout their lifetime (diverticulosis), whereas about one fifth may develop chronic abdominal symptoms including abdominal pain, bloating and changes in bowel habits, a condition termed symptomatic uncomplicated diverticular disease (SUDD). A minority of patients, about 1–4%, may develop acute diverticulitis, an inflammatory condition that may evolve in complications like abscesses, perforation, fistula, obstruction, or peritonitis [2]. Patients with a previous diverticulitis (PD) may experience recurrent diverticulitis in about 20% of cases and/or complain chronic-recurrent gastrointestinal symptoms in absence of inflammation [3, 4]. Simpson et al. show that about 67% of patients hospitalized for acute diverticulitis developed new, recurrent, short-lived abdominal pain following discharge [4]. Similarly, Cohen E et al. in a retrospective cohort study show that subjects with previous acute diverticulitis are 4.7 times more at risk for developing irritable bowel syndrome (IBS) than controls, configuring a condition called “post-diverticulitis IBS” [5].
Patients with symptomatic diverticular disease (SUDD and PD) may share a similar clinical pattern characterized by chronic-recurrent symptoms like abdominal pain, bloating and changes of bowel habits, difficult to differentiate from IBS. Symptoms attributable to diverticula overlapped with IBS in 6–60% of cases [6, 7] but it has been reported that some clinical characteristics may help in differentiating these conditions [8, 9]. For these reasons, an accurate characterization of abdominal pain in patients with diverticular disease (DD) can be very challenging in clinical practice.
Aim of this study was to assess the clinical features of abdominal pain, in terms of presence, severity and length, in patients with SUDD and PD using standardized questionnaires for DD and IBS.
Materials and methods
Study design
We utilized the REMAD registry, a 5-year, prospective, observational, multicentre, cohort study in which, a total of 1217 patients from 47 Italian centres were enrolled during the 2 months recruitment period and followed up for 5 years (from April 2015 to April 2020). Materials and methods were reported in previous papers [10,11,12]. Briefly, inclusion criteria were: informed consent; age \(\ge\) 18 years; endoscopic/radiological-confirmed colonic diverticula. Exclusion criteria were: failure to sign informed consent; inability to adhere to the study procedures.
Data collection
At entry, patients were categorized according to the following criteria: (i) diverticulosis, presence of diverticula in the absence of abdominal symptoms; (ii) SUDD, recurrent abdominal pain mainly in the lower abdominal quadrants, with a frequency of at least once a week, present for at least 6 months, and/or changes in bowel habit, without a well-defined previous attack of acute diverticulitis; (iii) PD, patients who experienced at least one episode of acute diverticulitis, complicated or not; when available medical charts were reviewed [10, 11]. Demographic and personal data, life-styles factors and assumption of drugs with gastrointestinal (GI) effect were collected. Patients who fulfilled criteria for diagnosis of SUDD and PD were asked to fill in questionnaires for characterization of abdominal pain: (i) questionnaire for short-lasting pain (< 24 h); (ii) questionnaire for long-lasting pain (> 24 h); (iii) questionnaire for irritable bowel syndrome (IBS) following Rome III Criteria. Patients with diverticulosis were not invited to complete the questionnaires as they were asymptomatic and were excluded from the current analysis. Questionnaires were administrated during outpatient’s visits, face to face, by dedicated physicians.
Abdominal short-lasting pain questionnaire
Short-lasting pain was defined as presence of episodes of abdominal pain lasting lesser than 24 h in the last 6 months. The questionnaire assessed: (i) pain’s abdominal localization (upper left, lower left, upper right, lower right and diffuse), (ii) the association with bowel habits (pain improved/not improved/partially improved by defecation), (iii) pain’s severity by means of a visual analogue scale (VAS) and semi quantitatively as mild (not influencing usual activities/causing minimal discomfort), moderate (clearly present and bothersome) or severe (not allowing normal daily activities) [4, 6, 13]. For abdominal pain localization, more than one abdomen section could be selected.
Abdominal long-lasting pain questionnaire
Long-lasting pain was defined as presence of episodes of abdominal pain persisting more than 24 h in the last 6 months. This questionnaire evaluated the presence of: (i) number of episodes of abdominal pain, (ii) abdominal pain’s localization (upper left, lower left, upper right, lower right and diffuse), (iii) if pain improves with the use of antispasmodics, (iv) severity of pain both by means of a VAS and semi quantitatively as mild (not influencing usual activities/causing minimal discomfort), moderate (clearly present and bothersome) or severe (not allowing normal daily activities). Moreover, some characteristics associated to long-lasting pain were investigated: presence of fever, confinement to bed, medical consultation, antibiotic therapy and hospital admission [4, 6, 13].
Questionnaire for irritable bowel syndrome
The diagnosis of IBS was based on the following criteria according to Rome III: presence of abdominal discomfort or pain at least 3 days/month in the last 3 months, with onset at least 6 months prior to diagnosis, associated with two or more of the following three characteristics: (i) relief with defecation, and/or (ii) onset associated with a change in frequency of stool, and/or (iii) onset associated with a change in aspect of stools [14]. IBS subtypes based on bowel habits: IBS-D (diarrhea predominant), IBS-C (constipation predominant), IBS-M (mixed predominant, alternating bowel habits), and IBS-U (unclassifiable bowel habits) were determined with the aid of the Bristol Stool Form Scale [15].
Statistical analysis
All data were first tested with the Shapiro Wilk test for checking normal distribution, and after were presented as counts and percentages when categorical variables and mean and standard deviation (SD) when continuous variables. The categorical variables were compared using the Chi-squared or Fisher’s exact tests as appropriate. For multiple categorical variables, the Chi-squared test of independence was used. The continuous variables were compared using the t test. The differences in demographics and clinical characteristics of patients with SUDD and PD cohorts were calculated. Subsequently, factors associated with SUDD versus PD diagnosis were explored using univariate and multivariate analysis. The estimated odds ratios (OR) with their 95% confidence intervals (CI) were calculated. The probability values were two-sided; a probability value of less than 0.05 was considered statistically significant. Statistical analysis was performed with STATA 17.0 (SE, Standard Edition, College Station, TX: StataCorp LP).
Results
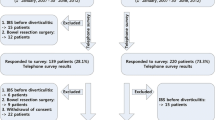
From the 1217 patients included in the REMAD registry, 705 patients (57.9%) fulfilled criteria for diagnosis of diverticulosis, 300 patients (24.7%) fulfilled criteria for SUDD and the remaining 212 patients (17.4%) fulfilled criteria for PD. Patients with diverticulosis (n = 705) were excluded from the current analysis. Among them, 148 out of 300 patients with SUDD and 118 out of 212 patients with PD completed all questionnaires, representing the study population (Fig. 1). In PD patients, diverticulitis was diagnosed in 78.9% with abdominal computed tomography and in 21.1% with abdominal ultrasound. 96.6% of patients with acute diverticulitis have available medical charts, whereas in 3.4% of patients the diagnosis was based on clinical and biochemical criteria by expert physicians. Differences regarding patient’s demographic and clinical characteristics between the SUDD and PD patients are reported in Table 1. Age was significantly lower in PD compared to SUDD patients (p = 0.027). There were no significant differences between patients in terms of gender and BMI. Regarding the main reason for DD diagnosis, abdominal pain was significantly more frequent in PD (p = 0.003), while bloating was only present in SUDD compared to PD patients (4.7% vs 0%, p = 0.019). Regarding lifestyle factors, active smokers were more frequent in PD compared to SUDD patients (p = 0.031), but no significant differences were found in terms of alcohol, coffee intake and physical activity.
Except for the use of mesalazine that was more frequent in PD patients (p = 0.001), no differences regarding the use of drugs with GI effect assumed in the previous 12 months was found.
Pattern of short-lasting and long-lasting abdominal pain
Table 2 shows the characteristics of short-lasting abdominal pain in SUDD and PD patients. Episodes of short-lasting abdominal pain, although highly prevalent in both groups, were significantly more frequently in SUDD than in PD patients (p = 0.007). No significant differences in terms of abdominal pain’s severity (by means of a VAS scale and semi quantitatively) or pain’s relief with defecation were found. Upper left abdomen localization was poorly observed, although more frequent in SUDD patients than PD (5.2% vs 1.1%, p < 0.001) (Fig. 2a).
Regarding long-lasting abdominal pain, differences between SUDD and PD groups are reported in Table 3. Presence of episodes of long-lasting abdominal pain was similar between SUDD and PD (59% vs 64%) but the number of episodes was significatively higher in SUDD (6.6 ± 11.9) compared to PD patients (3.4 ± 6.9) (p < 0.001). In terms of pain localization, PD patients reported long-lasting pain more frequently in the lower left abdomen (p < 0.001), while in SUDD it was more frequently diffuse (p = 0.002) or localized in the lower right quadrant (p = 0.009) (Fig. 2b). Moreover, features associated with long-lasting abdominal pain (fever, confinement to bed, consultations, antibiotic therapy, and hospitalization) were significantly more often reported in patients with PD than in patients with SUDD (Table 3). In patients using antispasmodics, pain improvement was similar between the two groups of patients.
Finally, considering together SUDD and PD patients, 132 out of 266 patients (49.6%) reported an overlap of short- and long-lasting abdominal pain, without significant differences between SUDD (n = 72, 54.5%) and PD (n = 60, 45.5%) (p = 0.805).
Univariate and multivariate analyses assessing pain-associated factors independently associated with PD diagnosis were performed as a post-hoc analysis (Table 4). At univariate analysis, we found a significant inverse association of PD diagnosis with the presence of short-lasting abdominal pain and the number of episodes of long lasting abdominal pain, while we found a positive correlation with lower left long lasting abdominal pain and its severity. At multivariate analysis only the lower left pain localization for long-lasting abdominal pain resisted as a predictive factor for PD diagnosis.
Overlap with irritable bowel syndrome
Diagnosis of IBS according to Rome III Criteria was reported in 75 patients (28.2%) and was more frequent in SUDD patients than in PD (37.2% vs 17.1%, p < 0.001) (Fig. 3a). Patients fulfilling the criteria for IBS diagnosis were stratified according to the bowel habits (IBS with diarrhoea, IBS with constipation, IBS mixed) (Fig. 3b). The most prevalent IBS subtype was the IBS-D, that was present in the 45 patients out of 75 with IBS (61.3%) [SUDD 33 (22.3%) vs. PD 12 (10.2%), p = 0.009], whereas IBS-C was found in 25 patients (33.3%) [SUDD 18 (12.2%) vs. PD 7 (5.9%), p = 0.084] and IBS-M in 5 patients (5.3%) [SUDD 4 (2.7%) vs. 1 (0.8%), p = 0.268].
Discussion
This observational multicentre cross-sectional study aimed to characterize abdominal pain in patients with symptomatic diverticular disease using standardized questionnaire for DD and IBS. In fact, it is matter of debate whether SUDD could be considered a disease its own or it represents the coexistence of IBS in patients with colonic diverticula; furthermore, after an episode of acute diverticulitis, patients may complain of chronic-recurrent abdominal pain, only sometimes related to a “low grade” recurrent diverticulitis [5].
Regarding short-lasting abdominal pain, we found that SUDD patients presented more often episodes of pain compared to PD patients, without significant differences in terms of pain severity or correlation of pain with defecation. At our knowledge, no study has directly compared SUDD and PD patients in terms of short-lasting abdominal pain. We found that about 90% of SUDD patients complained of short-lasting abdominal pain; this percentage seemed to be higher compared to 33–36% found, respectively, by Humes and Simpson [4, 16]. We believe that one of the main reason might be ascribed to the different way of questionnaires administration (postal in the cited two studies vs face-to-face interviews in our study) as recently reported [17]. However, previous studies from our group showed similar findings, with 70% and 82% of DD patients complaining of short-lasting abdominal pain [6, 13]. Regarding PD patients, Simpson et al. found that 69% of patients had short-lasting abdominal pain after a documented episode of acute diverticulitis, with a pain mean duration of 4 h and a pain intensity ranging from mild to moderate. This data might be considered in line with our findings, with 78% of PD patients complaining of short-lasting pain with a prevalence of moderate intensity in more than a third of patients (35.6%) [4].
Concerning the long-lasting abdominal pain (> 24 h), we found some interesting results. Although the proportion of SUDD and PD patients complaining of long-lasting pain was similar (58.8% vs 64.4%), the number of episodes was significantly higher in SUDD compared to PD. In current literature, the prevalence of long-lasting abdominal pain in patients with diverticular disease ranged from 5.6% to 100% [4, 6, 13, 16, 18] with no available data that directly compares SUDD and PD. We believe that there are at least two factors contributing to the wide observed range: first, some authors considered the presence of long-lasting pain as the main hallmark of SUDD diagnosis [18, 19] and others not [4, 6, 13]; second, often the population considered in these studies included patients with diverticulosis, potentially leading to misclassification bias. [4, 7, 16] We believe that considering as SUDD only patients presenting pain longer than 24 h may be limiting [20]; in fact, we demonstrated that more than 90% of SUDD patients complained of short-lasting abdominal pain, with about half of patients having also long-lasting pain. These data highlighted that abdominal pain regardless of its duration, is a remarkable symptom in this setting.
Moreover, we observed a different pattern of pain localization between SUDD and PD (Fig. 2a and b). In fact, we found that long-lasting abdominal pain was more frequently diffuse or localized in the lower right abdomen in SUDD patients than in PD (respectively, 32.2% vs 11.8%, p = 0.002 and 11.5% vs 1.3%, p = 0.009), while it was more frequently localized in the lower left abdomen in patients with PD (39.1% vs 81.6%, p < 0.001). Furthermore, at multivariate analysis only the lower left pain localization for long-lasting abdominal pain resisted as a predictive factor for PD diagnosis. Available data show that SUDD abdominal pain is predominantly localized in the lower left abdomen [8, 18, 21], while no studies assessed pain’s localization in PD patients. Furthermore, we showed that long-lasting abdominal pain was associated more frequently to occurrence of fever, confinement to bed, need for medical consultations, antibiotics therapies and hospitalization in patients with PD, than in SUDD. Considering together characteristics of short and long-lasting abdominal pain, these results suggested that SUDD complained symptoms more frequently than PD patients (i.e., higher occurrence of short lasting pain, higher number of episodes of long lasting pain), but with a less sever clinical phenotype (i.e., lower occurrence of fever, need of confinement to bed or need of antibiotic treatment), suggesting that in PD patients, chronic recurrent abdominal pain could sometimes underlying a possible recurrent diverticulitis. Another factor that might potentially contribute to this clinical scenario is smoking habit. Even if in the present study the number of active smokers is low, smokers are more frequent in PD compared to SUDD patients. A previous study showed that smoking was an independent factor associated with severe abdominal pain in IBS-M and IBS-C, regardless colonic transit time, supporting the hyphothesis that smoking might affect visceral perception and pain severity [22].
At now, even if the use of some biomarkers has been proposed for differential diagnosis between SUDD and IBS, such as fecal calprotectin [21, 23] or ultrasonography [24], no accepted criteria for SUDD diagnosis have been reached yet. In fact, only two small case–control studies, reported that fecal calprotectin levels (expressed semiquantitatively) are higher in SUDD compared to patients with IBS-like symptoms [21, 23], and no further larger studies are available. On the other hand, one study evaluated the role of intestinal ultrasound in discriminating SUDD among patients with abdominal symptoms. Patients with SUDD showed a significantly greater muscle thickness than those with IBS, patients with unclassifiable abdominal pain and healthy subjects, but comparable with those with diverticulosis [24]. Thus, available data are still scarce and then the clinical picture, particularly abdominal pain characteristics (length and pain localization), remained a reliable and largely used method to diagnose SUDD in clinical practice [6, 13, 21].
Chronic and recurrent abdominal symptoms associated to SUDD might be debilitating and might affect quality of life of patients [25]. In fact, some prospective studies have demonstrated an impairment in quality of life in SUDD patients compared to general population [26, 27]. Also, a more recent observational multicentre study has showed that quality of life in SUDD patients is similar to patients with a previous episode of diverticulitis, likely suggesting that the presence of troublesome recurrent abdominal symptoms is perceived a full disease similarly to patients who have experimented a diverticular complication [10].
Another interesting point is the current debate on the overlap between SUDD and IBS. In fact, some authors do not consider SUDD as a pathologic entity on its own, but merely the coexistence of IBS in patients with colonic diverticula [18, 21]. In our cohort we found that, according to Rome III Criteria, only about a third of SUDD patients fulfil criteria for IBS, suggesting that these patients can’t be all categorized as IBS, as two-thirds would remain outside this definition.
Another interesting finding in our study is the low occurrence of IBS-like symptoms in PD patients (17%), further suggesting that even after acute diverticulitis, which is a known predisposing condition, the overlap is weak. Previous data identified a wide overlap range between DD and IBS, from 6 to 60%, with high heterogeneity between studies, which in part might be due to the different criteria used to diagnose IBS (i.e., Rome II or Rome III Criteria) and also to the different spectrum of patients included (i.e., diverticulosis, SUDD or patients with previous diverticulitis, whether considered separately or not) [4, 6, 7, 13, 16, 18]. Regarding the IBS subtype, we found that IBS-D is the most common subtype, similarly to what previously reported [28]. In fact, a large population-based cross-sectional survey conducted by mailing and using Rome II Criteria, found that the presence of IBS was associated to DD (OR 1.7, 95% CI 1.2–2.4), especially the IBS-D (OR 1.9, 95% CI 1.1–3.2) [28].
The biological mechanisms that link DD and IBS-like symptoms are still matter of debate. It has been reported that alterations of intestinal microbiota, low grade inflammation and nerve sprouting may have a role in diverticular disease as in IBS [29,30,31]. In fact, as reported by Barbara et al. patients with colonic diverticular disease show depletion of microbiota members with anti-inflammatory activity (Clostridium cluster IV, Clostridium cluster IX, Fusobacterium and Lactobacillaceae) associated with mucosal macrophage infiltration [32]. Dysbiosis has often been suggest as an important player in SUDD pathogenesis and in symptoms generation [29]. Tursi et al. compared fecal microbiota of SUDD patients with both asymptomatic diverticulosis patients and healthy controls, showing a different composition of gut microbiota in diverticular disease patients compared to healthy controls [33]. Also, Kvasnovsky et al. showed that abdominal symptoms in SUDD patients might correlate with alterations in fecal microbiota, such as the severity of abdominal bloating which appears to associated with higher levels of bacteria involved in hydrogen production [34]. However, even if dysbiosis has been suggested as an important player in DD pathogenesis, only few and heterogenous studies are available and needed to be confirmed in further larger studies.
This study has some limitations. The main limit is the cross-sectional design, which does not allow to establish whether symptoms compatible with IBS were present before the detection of diverticula or not. In addition, the study has been designed in 2015, including Rome III instead of Rome IV criteria. In Rome III, a diagnosis of IBS included chronic abdominal pain or discomfort at least 3 days per month whereas in Rome IV the term discomfort has been removed and the pain frequency increased to at least 1 day per week [35]. This could lead to a possible overestimation of IBS diagnosis. Nevertheless, this is the first study that systematically assessed clinical features of abdominal pain, in terms of presence, severity and length, in patients with SUDD and PD using standardized questionnaires for DD and IBS in a large multicentric cohort.
We found that SUDD and PD patients presented different pattern of abdominal pain (i.e., pain’s length, number of long lasting pain episodes, localization and associated features), with only a third of the study population reporting an overlap with IBS. Further observational studies are needed to better characterize abdominal symptoms in patients with different spectrum of diverticular disease, especially in patients not fulfilling IBS diagnosis.
Data availability
Data supporting this study are available from the corresponding author upon reasonable request.
References
Stollman N, Raskin JB (2004) Diverticular disease of the colon. Lancet 363(9409):631–639. https://doi.org/10.1016/S0140-6736(04)15597-9
Shahedi K, Fuller G, Bolus R et al (2013) Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 11(12):1609–1613. https://doi.org/10.1016/j.cgh.2013.06.020
Bharucha AE, Parthasarathy G, Ditah I et al (2015) Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol 110(11):1589–1596. https://doi.org/10.1038/ajg.2015.302
Simpson J, Neal KR, Scholefield JH, Spiller RC (2003) Patterns of pain in diverticular disease and the influence of acute diverticulitis. Eur J Gastroenterol Hepatol 15(9):1005–1010. https://doi.org/10.1097/00042737-200309000-00011
Cohen E, Fuller G, Bolus R et al (2013) Increased risk for irritable bowel syndrome after acute diverticulitis. Clin Gastroenterol Hepatol 11(12):1614–1619. https://doi.org/10.1016/j.cgh.2013.03.007
Annibale B, Lahner E, Maconi G et al (2012) Clinical features of symptomatic uncomplicated diverticular disease: a multicenter Italian survey. Int J Colorectal Dis 27(9):1151–1159. https://doi.org/10.1007/s00384-012-1488-5
Kang JY, Firwana B, Green AE et al (2011) Uncomplicated diverticular disease is not a common cause of colonic symptoms. Aliment Pharmacol Ther 33(4):487–494. https://doi.org/10.1111/j.1365-2036.2010.04540.x
Spiller R (2012) Is it diverticular disease or is it irritable bowel syndrome? Dig Dis 30(1):64–69. https://doi.org/10.1159/000335721
Barbara G, Cremon C, Bellini M et al (2023) Italian guidelines for the management of irritable bowel syndrome: joint consensus from the italian societies of: gastroenterology and endoscopy (SIGE), neurogastroenterology and motility (SINGEM), hospital gastroenterologists and endoscopists (AIGO), digestive endoscopy (SIED), general medicine (SIMG), gastroenterology, hepatology and pediatric nutrition (SIGENP) and pediatrics (SIP). Dig Liver Dis 55(2):187–207. https://doi.org/10.1016/j.dld.2022.11.015
Carabotti M, Cuomo R, Barbara G et al (2018) Demographic and clinical features distinguish subgroups of diverticular disease patients: results from an Italian nationwide registry. United Eur Gastroenterol J 6(6):926–934. https://doi.org/10.1177/2050640618764953
Cremon C, Carabotti M, Cuomo R et al (2019) Italian nationwide survey of pharmacologic treatments in diverticular disease: results from the REMAD registry. United Eur Gastroenterol J 7(6):815–824. https://doi.org/10.1177/2050640619845990
Carabotti M, Morselli Labate AM, Cremon C et al (2021) Distinguishing features between patients with acute diverticulitis and diverticular bleeding: results from the REMAD registry. Dig Liver Dis 53(2):202–209. https://doi.org/10.1016/j.dld.2020.05.045
Cuomo R, Barbara G, Andreozzi P et al (2013) Symptom patterns can distinguish diverticular disease from irritable bowel syndrome. Eur J Clin Invest 43(11):1147–1155. https://doi.org/10.1111/eci.12152
Drossman DA, Dumitrascu DL (2006) Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 15(3):237–241
Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32(9):920–924. https://doi.org/10.3109/00365529709011203
Humes DJ, Simpson J, Neal KR et al (2008) Psychological and colonic factors in painful diverticulosis. Br J Surg 95(2):195–198. https://doi.org/10.1002/bjs.5962
Sperber AD, Bor S, Fang X et al (2023) Face-to-face interviews versus Internet surveys: comparison of two data collection methods in the Rome foundation global epidemiology study: Implications for population-based research. Neurogastroenterol Motil 35(6):e14583. https://doi.org/10.1111/nmo.14583
Peery AF, Keku TO, Addamo C et al (2018) Colonic diverticula are not associated with mucosal inflammation or chronic gastrointestinal symptoms. Clin Gastroenterol Hepatol 16(6):884-891.e1. https://doi.org/10.1016/j.cgh.2017.05.051
Tursi A, Franceschi M, Elisei W et al (2021) The natural history of symptomatic uncomplicated diverticular disease: a long-term follow-up study. Ann Gastroenterol 34(2):208–213. https://doi.org/10.20524/aog.2020.0560
Tursi A, Elisei W, Franceschi M et al (2021) The prevalence of symptomatic uncomplicated diverticular disease could be lower than expected: a single-center colonoscopy-based cohort study. Eur J Gastroenterol Hepatol 33(1S Suppl 1):e478–e483. https://doi.org/10.1097/MEG.0000000000002142
Tursi A, Elisei W, Picchio M, Giorgetti GM, Brandimarte G (2015) Moderate to severe and prolonged left lower-abdominal pain is the best symptom characterizing symptomatic uncomplicated diverticular disease of the colon: a comparison with fecal calprotectin in clinical setting. J Clin Gastroenterol 49(3):218–221. https://doi.org/10.1097/MCG.0000000000000094
Rurgo S, Vaino V, Andreozzi M et al (2022) Predictors of abdominal pain severity in patients with constipation-prevalent irritable bowel syndrome. J Basic Clin Physiol Pharmacol 33(5):665–671. https://doi.org/10.1515/jbcpp-2022-0081
Tursi A, Brandimarte G, Elisei W et al (2009) Faecal calprotectin in colonic diverticular disease: a case-control study. Int J Colorectal Dis 24(1):49–55. https://doi.org/10.1007/s00384-008-0595-9
Maconi G, Dell’Era A, Flor N et al (2023) Ultrasonographic and functional features of symptomatic uncomplicated diverticular disease. Clin Transl Gastroenterol 14(6):e00580. https://doi.org/10.14309/ctg.0000000000000580
Calini G, Abd El Aziz MA, Paolini L et al (2023) Symptomatic uncomplicated diverticular disease (SUDD): practical guidance and challenges for clinical management. Clin Exp Gastroenterol 16:29–43. https://doi.org/10.2147/CEG.S340929
Comparato G, Fanigliulo L, Aragona G et al (2007) Quality of life in uncomplicated symptomatic diverticular disease: is it another good reason for treatment? Dig Dis 25(3):252–259. https://doi.org/10.1159/000103896
Spiegel BM, Reid MW, Bolus R et al (2015) Development and validation of a disease-targeted quality of life instrument for chronic diverticular disease: the DV-QOL. Qual Life Res 24(1):163–179. https://doi.org/10.1007/s11136-014-0753-1
Jung HK, Choung RS, Locke GR 3rd et al (2010) Diarrhea-predominant irritable bowel syndrome is associated with diverticular disease: a population-based study. Am J Gastroenterol 105(3):652–661. https://doi.org/10.1038/ajg.2009.621
Marasco G, Buttitta F, Cremon C et al (2023) The role of microbiota and its modulation in colonic diverticular disease. Neurogastroenterol Motil 35(12):e14615. https://doi.org/10.1111/nmo.14615
Barbaro MR, Cremon C, Fuschi D et al (2019) Nerve fiber overgrowth in patients with symptomatic diverticular disease. Neurogastroenterol Motil 31(9):e13575. https://doi.org/10.1111/nmo.13575
Barbaro MR, Cremon C, Fuschi D et al (2022) Pathophysiology of diverticular disease: from diverticula formation to symptom generation. Int J Mol Sci 23(12):6698. https://doi.org/10.3390/ijms23126698
Barbara G, Scaioli E, Barbaro MR et al (2017) Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut 66(7):1252–1261. https://doi.org/10.1136/gutjnl-2016-312377
Tursi A, Mastromarino P, Capobianco D et al (2016) Assessment of fecal microbiota and fecal metabolome in symptomatic uncomplicated diverticular disease of the colon. J Clin Gastroenterol 50(Suppl 1):S9–S12. https://doi.org/10.1097/MCG.0000000000000626
Kvasnovsky CL, Leong LEX, Choo JM et al (2018) Clinical and symptom scores are significantly correlated with fecal microbiota features in patients with symptomatic uncomplicated diverticular disease: a pilot study. Eur J Gastroenterol Hepatol 30(1):107–112. https://doi.org/10.1097/MEG.0000000000000995
Aziz I, Törnblom H, Palsson OS et al (2018) How the change in IBS criteria from Rome III to Rome IV impacts on clinical characteristics and key pathophysiological factors. Am J Gastroenterol 113(7):1017–1025. https://doi.org/10.1038/s41395-018-0074-z
Acknowledgements
This work was supported by unconditional funding by Alfasigma, Italy. REMAD group: Alida Andrealli, U.O. Gastroenterology Valduce Hospital Como; Sandro Ardizzone, Endoscopy Service A.O. Fatebenefratelli and Ophthalmic, Milano; Marco Astegiano, SSCVD Diagnosis and Treatment of Intestinal Diseases AOU Città della Salute e della Scienza, Torino; Francesco Bachetti, Department of Medicine, University of Perugia; Simona Bartolozzi, SC Gastroenterology and Digestive Endoscopy ASL Novara – PO Borgomanero; Stefano Bargiggia, UO Gastroenterology, PO “A. Manzoni”, Lecco; Gabrio Bassotti, Department of Medicine, University of Perugia; Maria Antonia Bianco, UOC Gastroenterology and Digestive Endoscopy ASL NA3sud—Maresca Hospital Torre del Greco; Giuseppe Biscaglia, U.O.C. Gastroenterology and Digestive Endoscopy Casa Sollievo della Sofferenza—IRCCS Opera di San Pio da Pietrelcina San Giovanni Rotondo; Matteo Bosani, Gastroenterology Unit, General Hospital Legnano, Legnano; Maria Erminia Bottiglieri, UOC Gastroenterology and Digestive Endoscopy, PO Marcianise; Martina Cargiolli Gastroenterology Department of Clinical Medicine and Surgery, School of Medicine “Federico II”, Napoli; Carolina Ciacci, Department of Medicine, Surgery and Dentistry, University of Salerno; Antonio Colecchia, UO Gastroenterology, AOU Borgo Trento Hospital of Verona; Agostino Di Ciaula, Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro; Alessandra Dell’Era, Division of Gastroenterology L. Sacco Hospital Milan; Marina De Matthaeis, S.S.D. Gastroenterology Emergency Endoscopy ASL 4 Chiavarese—Lavagna Hospital, Lavagna; Mirko Di Ruscio, Gastroenterology Unit, IRCCS Ospedale Sacro Cuore-Don Calabria, Verona; Marco Dinelli, Endoscopy Unit, San Gerardo Hospital, ASST Monza; Virginia Festa, UOC Gastroenterology and Hepatology, ACO San Filippo Neri, Roma; Ermenegildo Galliani, U.O.C. Gastroenterology Azienda ULSS 1 San Martino Hospital Belluno; Bastianello Germanà, U.O.C. Gastroenterology Azienda ULSS 1 San Martino Hospital Belluno; Mario Grassini, SOC Gastroenterology and Digestive Endoscopy, Cardinal Massaia Hospital, Asti; Ennio Guido, UOC Gastroenterology, S. Antonio Hospital, Padova; Franco Iafrate, Department of Radiological, Oncological and Pathological Sciences, Sapienza University, Roma; Paola Iovino, Department of Medicine, Surgery and Dentistry, University of Salerno; Donato Iuliano, UOC Gastroenterology and Digestive Endoscopy, PO Marcianise; Andrea Laghi, Department of Medical-Surgical Sciences and Translational Medicine, Sapienza University, Roma; Giovanni Latella, Department of Life, Health and Environmental Sciences, University of L’Aquila; Gianpiero Manes, Gastroenterology Service, AOG Salvini, Garbagnate Milanese; Elisa Marabotto, Department of Internal Medicine, University of Genova; Alessandro Moscatelli, Lavagna S.S.D. Gastroenterology Emergency Endoscopy—Lavagna Hospital; Riccardo Nascimbeni, Brescia; Department of Molecular and Translational Medicine University of Brescia, Civil Hospitals of Brescia; Pietro Occhipinti, Medical Department of Gastroenterology AOU Maggiore della Carità Novara; Marco Parravicini, Gastroenterology and Digestive Endoscopy Unit, AOU Circolo di Varese; Marco Pennazio, SC Gastrohepatology U, AOU City of Health and Science, Torino; Sergio Peralta,Digestive Endoscopy AOU Policlinico Paolo Giaccone, Palermo; Piero Portincasa, Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro; Franco Radaelli, U.O. Gastroenterology Valduce Hospital Como; Raffaella Reati, Gastroenterology Service, AOG Salvini, Garbagnate Milanese; Alessandro Redaelli, Endoscopy Unit, San Gerardo Hospital, ASST Monza; Marco Rossi, SC of Gastroenterology and Digestive Endoscopy, San Donato Hospital, Arezzo; Raffale Salerno, Milano; Endoscopy Service A.O. Fatebenefratelli and Ophthalmic Milan; Sergio Segato, SC Gastroenterology and Digestive Endoscopy, Macchi Foundation Circolo Hospital, Varese; Carola Severi, Department of Translational and Precision Medicine, Sapienza University, Roma; Giuseppe Scaccianoce, Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro; Valentina Valle, S.C. General and Hepatobiliopancreatic Surgery E.O. Galliera hospitals, Genova; Clara Virgilio, U.O.C. Gastroenterology ARNAS Garibaldi Catania; Angelo Viscido, Department of Life, Health and Environmental Sciences, University of L’Aquila.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. No funding for this article.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Coordinator Centre (University Federico II, Naples n. 161/14, 24 September 2014), and the centre local ethical approvals obtained from the other centres.
Human and animal rights
All procedures were approved by Ethics Committee.
Informed consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The complete details of author involved in REMAD group are given in acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carabotti, M., Marasco, G., Sbarigia, C. et al. Site and duration of abdominal pain discriminate symptomatic uncomplicated diverticular disease from previous diverticulitis patients. Intern Emerg Med (2024). https://doi.org/10.1007/s11739-024-03588-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11739-024-03588-6