Abstract
Epicardial adipose tissue is a novel cardiometabolic risk factor and indicator of subclinical atherosclerosis. We aimed to evaluate the epicardial adipose tissue thickness in rheumatoid arthritis (RA) patients and its association with disease activity scores. A total of 81 rheumatoid arthritis patients and 70 age- and sex-matched healthy individuals were recruited for this cross-sectional study. Epicardial adipose tissue thickness (EATT) was measured by transthoracic two-dimensional echocardiography. Tender and swollen joint counts were recorded at the time of inclusion. The laboratory tests included erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor, anti-citrullinated protein antibodies, and serum lipid levels. Disease activity was calculated based on Disease Activity Scores for 28 joints (DAS-28) ESR and CRP, the Simple Disease Activity Index (SDAI), and the Clinical Disease Activity Index (CDAI). Epicardial adipose tissue thickness was significantly higher in the RA patients compared to the healthy controls (p < 0.001). We found statistically significant correlations of EATT with all disease activity indices (p < 0.001) and CRP (p = 0.002). According to a cut-off value of 6.4 mm determined for epicardial adipose tissue thickness, the RA patients with thickness ≥ 6.4 mm had higher disease activity scores and CRP levels. In the multivariable regression analysis, only SDAI score was found as an independent risk factor for increased EATT (OR, (95%CI), 13.70 (3.88–48.43), p < 0.001). Epicardial adipose tissue thickness measurement by echocardiography is a reliable method for assessing subclinical atherosclerosis in rheumatoid arthritis patients, and a higher disease activity score is an independent risk factor for coronary artery disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease with a prevalence of 0.5–1% resulting in progressive joint damage [1]. It not only affects the synovial joints but also presents with extra-articular manifestation, and most importantly, is associated with increased morbidity and mortality [2, 3]. The leading cause of increased mortality in RA patients is the cerebrocardiovascular events [4]. Autoimmunity and systemic inflammation have the major contribution to the increased rates of adverse cardiovascular events [1, 5, 6].
The progression of atherosclerosis is multifactorial, and inflammation also has an important role [7]. It was reported that people with RA who have persistent inflammation are more likely to develop subclinical atherosclerosis [7]. In a prospective study, it was shown that failure to adequately control RA activity increases the risk of developing subclinical atherosclerosis, and cardiovascular events four to eight times [2]. Inflammation markers such as the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), tender joint counts (TJC), swollen joint counts (SJC), the presence of a deformity, and extra-articular disease manifestations have been demonstrated to be associated with an increased risk of atherosclerosis [8].
Carotid-intima-media thickness and the presence of carotid plaques have been shown as reliable predictors of subclinical atherosclerosis, and to be increased in many inflammatory rheumatic diseases including RA [3, 9,10,11]. Nevertheless, epicardial adipose tissue (EAT) is a novel cardiometabolic risk factor, and an indicator of atherosclerosis [12].The epicardial adipose tissue is located on the free wall of the right ventricle and the left ventricular apex between the visceral pericardium, and the myocardium surrounding the subepicardial coronary arteries [11]. It is a complex endocrine organ that secretes many proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, tumor-necrosis factor, and proatherogenic factors in excessive amounts [13]. Studies have shown that EAT plays a role in the initiation and progression of coronary artery disease (CAD) due to its close anatomical location to the adventitia of coronary arteries and its paracrine and local inflammatory effects [14, 15]. Epicardial adipose tissue thickness (EATT) was demonstrated to be associated with atherosclerotic plaques, and an independent predictor of CAD [16]. Although the radiological measurement of EAT thickness provides a more accurate result, transthoracic echocardiographic (TTE) measurement is still an accurate, inexpensive, non-invasive, and easily accessible method [12].
In this study, we aimed to evaluate EAT thickness in RA patients without prior CAD and its association with disease activity indices to determine the risk of subclinical atherosclerosis in RA patients.
Patients and methods
Study population
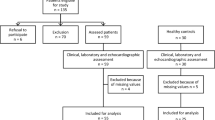
Patients aged ≥ 18 years, diagnosed with RA according to the 2010 American College of Rheumatology/European League Against Rheumatism Rheumatoid Arthritis Classification Criteria, who presented to a tertiary rheumatology outpatient clinic between May 2022 and September 2022 were included in the study [17]. Pregnant or breast-feeding patients, and patients with known concurrent comorbidities like arterial hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disease, chronic liver or kidney diseases, solid or hematological malignancies, acute or chronic infections, or other autoimmune/autoinflammatory diseases were excluded from the study. Healthy control (HC) group was selected from patients who admitted to the rheumatology outpatient clinic and were examined thoroughly regarding any rheumatic disease. Patients who were not diagnosed with any autoimmune and/or autoinflammatory rheumatic diseases as well as the other exclusion criteria diseases stated above according to patient history, physical examination and laboratory test results were also recruited for the study. The control group was randomly selected by stratification according to age and sex.
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the institution (Number:109/37, Date:19.04.2021). Written informed consent was obtained from all participants.
Demographic and clinical characteristics
Information on the demographic and clinical characteristics of the participants was obtained at the time of recruitment. Their disease onset, smoking status, treatment characteristics, Tender joint count (TJC), swollen joint count (SJC), and patient’s and physician’s visual analogue scale assessments (0–100 cm) were recorded. The body mass index (BMI) was calculated as body weight divided by height squared (kg/m2). Blood samples were collected in the morning after 8 h fasting, and fasting glucose, total cholesterol, low-density lipoprotein (LDL), triglyceride (Tg), and high-density lipoprotein (HDL) levels were measured. Other laboratory tests included hepatic transaminases, serum creatinine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), anti-citrullinated protein antibody (ACPA), and complete blood count (CBC).
Assessment of rheumatoid arthritis disease activity
The disease activity assessments for the RA patients were performed according to disease activity scores—ESR based on 28-joints (DAS28-ESR), DAS28-CRP, the Simplified Disease Activity Index (SDAI), and the Clinical Disease Activity Index (CDAI) [18,19,20].
Transthoracic echocardiography
The RA patients and healthy controls underwent detailed transthoracic two-dimensional imaging, and tissue Doppler imaging echocardiography procedures were performed with a Philips EPIQ 7 device (Philips Ultrasound; Bothel, WA, USA) by standard techniques [21, 22]. The echocardiographic examinations were performed by a cardiology specialist, and the evaluation was blinded. Routine echocardiographic measurements were obtained according to the recommendations of the American Society of Echocardiography [23]. For the epicardial adipose tissue measurements, the patient was placed in the lateral decubitus position, and optimal parasternal long-axis images were obtained. For optimization, the aortic root was determined as the reference, and the right ventricular free wall and aortic annulus were ensured to be in the midline [24, 25]. Epicardial adipose tissue is defined as an echo-free space between the outer border of the myocardial wall and the visceral layer of the pericardium. After long-axis imaging, EAT measurements were made in the mid-chordal section by short-axis imaging. The measurements were made in three cardiac cycles, their mean value was calculated, and the data included the end-diastole part, the section taken just before the R wave in the ECG [26].
Statistical analysis
IBM SPSS Statistics for Windows, version 26.0 (SPSS Inc, Chicago, IL, USA) was used to perform the statistical analyses. The variables were investigated using visual (histograms, probability plots) and analytical (Kolmogorov–Smirnov/Shapiro–Wilk test) to determine whether they were normally distributed. Due to non-normally distribution of the continuous variables, they were presented as median and IQR values. The categorical variables are presented as frequency values. Since non-normally distribution was observed for numerical continuous variables in the intergroups comparisons, Mann–Whitney U-test was used and for categorical variables chi-squared or Fischer’s test were used when appropriate. Correlation analysis between epicardial adipose tissue thickness and disease activity indices was performed using Spearman’s test. In order to determine the independent risk factors affecting epicardial adipose tissue thickness, variables with p < 0.25 in univariate analyzes and traditional cardiovascular risk factors were taken into account. Among these variables, when the correlation coefficient between them was about 0.6, the one with the larger p value or a lower clinical significance was excluded from the study. The remaining variables were included in the logistic regression analysis by the enter method. Odds ratios (OR) and their 95% confidence intervals were calculated for the independent risk factors identified. Model accuracy was evaluated with the Hosmer–Lemeshow goodness of fit statistics. Power analyses were performed using the G* Power version 3.0.10 power and sample size calculation program. A p-value < 0.05 was accepted as statistically significant.
Results
A total of 81 RA patients and 70 age- and sex-matched HC were enrolled in this case–control study. Both groups were similar in terms of their BMI values and smoking status. None of the patients or the HC were on lipid-lowering drugs and in both groups, blood lipid levels were within normal range (i.e., LDL < 160 mg/dL, Tg < 150 mg/dL, HDL > 40 mg/dL). The demographic characteristics and laboratory results of the RA patients and HC are summarized in Table 1. The RF and ACPA parameters were positive in 79% and 69% of the RA patients, respectively. Methotrexate (MTX) was the most commonly used disease-modifying anti-rheumatic drug (DMARD) at a rate of 62% among the RA patients, which was followed by leflunomide (LEF) in 27%, hydroxychloroquine sulfate (HCQ) in 54%, and biological DMARD in 6%. The majority of the patients had moderate disease activity according to their DAS28-ESR, DAS28-CRP, and SDAI scores. The epicardial adipose tissue thickness values of the RA patients were significantly higher compared to the HC (p < 0.001). The demographic, clinical, laboratory and treatment characteristics of RA and HC are summarized in Table 1.
The EATT results of the RA patients showed strong, positive and significant correlations with their DAS28-ESR (rho: 0.778, p < 0.001), DAS28-CRP (rho: 0.882, p < 0.001), SDAI (rho: 0.835, p < 0.001) and CDAI (rho: 0.838, p < 0.001) results (Fig. 1). The EATT also showed a moderate correlation with CRP (rho: 0.344, p = 0.002). On the other hand, no statistically significant correlation was found between EATT, RA disease duration, age and body mass index (p = 0.331, p = 0.435, and p = 0.103, respectively). Table 2 summarizes the association of EATT with disease activity indices, disease duration, age, and BMI.
The RA patients were divided into two groups as the remission and low disease activity group and the moderate-to-high disease activity group based on their disease activity index values (DAS28-ESR, DAS-28-CRP, SDAI, and CDAI). The EATT was found significantly higher in the moderate-to-high disease activity group compared to the remission-low disease activity group in all four disease activity indices (Table 3).
The median EATT among the RA patients was found as 6.4 mm. When this value was used as the cut-off value, the EATT ≥ 6.4 was accepted as ‘increased EATT’. The RA patients were then sub-grouped as patients with EATT < 6.4 (n = 39) and those with EATT ≥ 6.4 (n = 42). The median age, sex, disease duration, BMI, smoking status, and seropositivity results were not significantly different between the groups (p = 0.526, p = 0.246, p = 0.285, p = 0.087, p = 0.573, p = 0.823, and p = 0.434, respectively). Nevertheless, CRP levels were significantly higher in the patients with EATT ≥ 6.4 compared to the patients with EATT < 6.4 (p = 0.007). Likewise, the disease activity scores of the RA patients with increased EATT were significantly higher in comparison to the RA patients with EATT < 6.4 (Table 4).
We analyzed the independent variables that had the greatest impact on higher EATT (i.e.; EATT ≥ 6.4) further using logistic regression models. The components with a p-value < 0.100 and the components of metabolic syndrome were included in the analysis. In the multivariate analysis, SDAI was found to be an independent risk factor for increased EATT (p < 0.001) (Table 5).
Discussion
This study demonstrated that EATT was increased in the RA patients compared to the healthy controls as a predictor of subclinical atherosclerosis. Additionally, EATT was correlated with the disease activity scores of the patients with RA that include DAS28-ESR, DAS28-CRP, SDAI, and CDAI. This study also showed that only the increased disease activity score was an independent risk factor for increased EATT in RA patients. To the best of our knowledge, although, there are a few studies in the literature evaluating EATT in RA patients with contradictory results about its association with disease activity, this is the first study that included SDAI and CDAI as novel disease activity markers of RA and found a significant relationship between disease activity and increased EATT.
Epicardial adipose tissue is a true visceral adipose tissue surrounding the subepicardial coronary arteries [24]. It is a metabolically active paracrine and endocrine organ secreting many proinflammatory and proatherogenic cytokines, chemokines, and adipokines [27]. In addition to systemic effects, due to its direct contact with coronary arteries, it may also directly contribute to coronary atherogenesis [28]. Accordingly, EAT plays a role in the development of subclinical atherosclerosis through vascular inflammation and endothelial dysfunction [29]. Several studies have revealed the association of EAT with adverse cardiovascular events ranging from asymptomatic to overt coronary artery disease independently of other risk factors [30]. In recent years, it was shown that the pathogenesis of atherosclerosis is a dynamic process in which inflammation plays a role in all stages [7]. This study showed that EATT increases in patients with RA, which is a chronic inflammatory disease, as a predictor of subclinical atherosclerosis. Likewise, in the studies conducted by Temiz et al. and Fatma et al., EATT was significantly higher in RA patients in comparison to HC [31, 32]. Lima-Martinez et al. conducted a study with 34 female RA patients and showed that healthy women had the lowest EATT values compared to RA patients, and RA patients receiving biological DMARDs had lower EATT in comparison to patients receiving non-biological DMARDs [33]. In the study by Başpınar et al., the epicardial fat area measured by computed tomography was significantly higher in RA patients in comparison to healthy individuals [34]. On the other hand, in the study by Ormseth et al., EATT was not significantly higher in RA patients in comparison to HC (p = 0.06), although RA patients with metabolic syndrome (n = 56) had significantly higher EATT values compared to RA patients without metabolic syndrome (n = 101) [35]. There are a few studies in the literature that have evaluated EATT in other rheumatic diseases in which increased EATT values have been demonstrated in patients with systemic sclerosis, systemic lupus erythematosus (SLE), ankylosing spondylitis, and inflammatory bowel disease. [36,37,38,39]
Corticosteroids have major effects on adipose tissue metabolism via glucocorticosteroid specific receptors which are at the highest density in the visceral adipose tissue. It has been shown that high doses of steroid increase the visceral adipose tissue [40]. Besides, the long-term steroid use is associated with weight gain, even obesity, which in return causes metabolic syndrome, and are related with the features of metabolic syndrome. Kitterer et al., performed the first study that evaluated the effect of long-term steroid therapy on epicardial and pericardial fat deposition. Their study included 61 patients with various rheumatic diseases (mainly vasculitis), and age-sex-BMI matched healthy controls. Larger epicardial fat was found in patients treated with steroids in comparison to steroid-naϊve patients. In addition, significantly higher epicardial fat was found in patients on high-dose steroids (defined as > 7.5 mg prednisone equivalent per day for at least 6 months) compared to patients on low-dose streoids (ie., < 7.5 mg prednisone equivalent per day for at least 6 months). However, no significant difference was observed in epicardial and pericardial adipose fat between patients on low-dose steroids and steroid-naϊve [40]. In the cross-sectional study that included 162 SLE patients and 86 healthy controls, EAT was significantly higher in SLE patients than in controls, and when adjusted for age, sex, race, and waist circumference, only cumulative corticosteroid dose was remained significantly associated with the EAT volume [37]. Even though, corticosteroid use may have had an influence on higher EATT in RA patients in comparison to healthy controls in our study, the majority of our patients were on low-dose steroid (median 5 mg, [IQR, 5–7.5]). Likewise, in the study of Karpouzas et al., 139 RA patients who underwent coronary angiography for EAT measurement and coronary plaque assessment, the EAT volume was found higher in patients with atherosclerosis compared to patients without atherosclerosis, and it was related with the number of segments with plaque as well as mixed plaque presence in RA patients. Yet, they demonstrated that prednisone use did not alter the association between the EAT volume and the plaque burden [41].
Moreover, we showed that EATT was significantly correlated with disease activity scores, and in the logistic regression analysis, only disease activity was demonstrated to be an independent risk factor for increased EATT. Besides EAT’s contribution to systemic inflammation, systemic inflammation in return causes adipogenesis leading to an accumulation of fat in epicardial tissue promoting a positive feed-back on inflammation and accelerated atherosclerosis [28, 41]. Therefore, an increase in EATT is expected to be seen in RA patients with higher disease activity scores for which we found a cut-off value of 6.4 mm, and patients with EATT ≥ 6.4 mm in our study were found to have higher DAS28-ESR, DAS28-CRP, SDAI, and CDAI scores. Data about the correlation of EATT with disease activity in rheumatic diseases are insufficient and conflicting. Similar to our study, in the study by Alpaydın et al., DAS28 scores were demonstrated to be positively correlated with EATT (r = 0.365, p = 0.02) [42]. In a study conducted with 115 psoriasis patients, higher EATT values were found in the patients in comparison to healthy individuals, and EATT was correlated with psoriasis disease activity scores [43]. Psoriasis Area and Severity Index scores were also shown as an independent predictor of EAT [43]. Again, in a study conducted with systemic sclerosis patients (n = 30), EATT was demonstrated to be correlated only with disease activity scores (r = 0.45, p = 0.01) [44]. Contrary to our results, Petra et al. and Ormseth et al. did not find any significant correlations between ESR, CRP, DAS28-CRP scores, and EATT values [35, 45]. Temiz et al. showed significant correlations of EATT with ESR, CRP, and Health Assessment Questionnaire scores, but EATT was not correlated with DAS28 scores [31]. Nonetheless, we believe that, as the strength of our study, we showed the correlation of EATT not only with DAS28 but also with other novel disease activity scores of RA, namely SDAI and CDAI.
Another noteworthy finding of our study was that in the univariate and multivariate regression analyses, we found SDAI as an only predictor of increased EATT, and therefore, an independent risk factor for subclinical atherosclerosis in RA patients. As far as we know, this is the first study that showed higher disease activity as a risk factor for EATT. In our study, we did not observe any correlation between EATT and disease duration. Similar results were also found in other studies which reported that the progression of the disease does not result in a significant increase in EATT [31, 45]. These results may be interpreted as disease activity, rather than disease duration, has a greater influence on increased risk of cardiovascular events, even in the early stages of the disease.
There is copious amount of data regarding the association of EATT with coronary atherosclerosis, yet, the number of prospective large-population based studies on the prognostic value of increased EATT for the prediction of future major adverse cardiac events (MACE) are limited. In a prospective study in which 200 patients with a clinical diagnosis of CAD who underwent left and right coronary angiography were followed-up for 26 months, the incidence of death due to cardiovascular events was significantly higher in patients with EATT > 7 mm [46]. The Heinz-Nixdorf Recall study is a prospective population cohort that evaluated the predicted value of EAT volume for coronary events. Of the 4,093 participants without a baseline coronary heart disease, during a follow-up year of 8.0 ± 1.5 years, 130 of them had fatal or non-fatal coronary events developed. The EAT volume was found higher in subjects who developed any coronary event (121 mL vs 95 mL, p < 0.001). The study also showed that the incident of coronary events increased with the EAT volume, and the subjects in the highest EAT volume quartile had a fivefold higher risk of having a coronary event in comparison to subjects in the lowest EAT volume quartile [47]. Again, in a recent study that included 2068 asymptomatic participants without a prior coronary artery disease from the prospective, randomized EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research), MACE were reported in 223 (11%) of participants during a mean follow-up of 13.9 ± 3 years. By using deep-learning which is a subset of machine-learning, the EAT volume and attenuation were quantified. The EAT volume was shown to be significantly higher in participants with MACE than without MACE (90.6 [IQR, 67.4–128.7] cm3 vs. 77.0 [54.7–103.3] cm3, p < 0.001). In multivariate analysis, adjusted after atherosclerotic cardiovascular disease risk score, the EAT volume was found to be associated with increased risk of MACE (HR, 1.35 [95% CI, 1.07–1.68], p = 0.009). Again, there was a strong correlation between the EAT volume and the subsequent myocardial infarction and cardiac mortality (HR, 1.49 [95% CI, 1.00–2.21], p = 0.046) [48]. On the contrary, Albuquerque et al. evaluated 194 patients with a known coronary artery disease who entered a phase II cardiac rehabilitation program for a mean follow-up of 3.6 ± 1.3 years, and they showed that the EAT when measured by echocardiography was not a predictor of MACE (HR, 1.32 [95% CI, 0.75–2.31, p = 0.33]); which was defined as diagnosis of acute coronary syndrome, coronary revascularization, stroke, ventricular arrhythmias requiring hospitalization and death of any cause [49]. However, this study had some limitations such as shorter follow-up period when compared to other studies, smaller study population and the presence of higher comorbid diseases of the patients. Furthermore, as the study population included patients with a known previous coronary artery disease, this study may be limited to explain the role of EATT for subclinical atherosclerosis and the future cardiovascular events.
The main limitations of our study were its cross-sectional design and the lack of a measurement of arterial blood pressure and waist circumference. However, as we excluded patients with other cardiovascular risk factors such as diabetes mellitus, hypertension, and hyperlipidemia, we could make a more accurate analysis of predictors influencing EATT. Another limitation of this study may be the use of two-dimensional echocardiography to measure EATT as its sensitivity may be lower especially in obese patients and that two-dimensional measurement may not fully assess the entirety of EAT. Yet, a good correlation of echocardiographic EAT with magnetic resonance imaging measurements was previously shown [50]. Echocardiography is still an easily accessible and inexpensive method for measuring EATT. Again, all echocardiographic measurements in this study were performed by a single cardiologist, which might be another limitation of this study. The last limitation of our study was the lack of assessing plaque burden and plaque types in coronary arteries as well as different arterial sites by imaging methods such as computed tomography angiography or vascular ultrasound which could have improved our results of the association of EATT with subclinical atherosclerosis.
Conclusions
The epicardial adipose tissue thickness is an emerging cardiometabolic risk factor, and its measurement by echocardiography is a reliable and an easy method for predicting subclinical atherosclerosis in RA patients. Due to its correlation with disease activity and the finding that a higher disease activity score is an independent risk factor for higher EATT, the tight control of disease activity in RA reduces not only the risk of joint damage and disability but also the risk of adverse cardiovascular events and mortality in RA patients. Larger, prospective and mechanistic studies are needed to elucidate the association between EATT and atherosclerosis in patients with rheumatoid arthritis.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Spinelli FR, Pecani A, Ciciarello F, Colasanti T, Di Franco M, Miranda F et al (2017) Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet Disord 18:1–9. https://doi.org/10.1186/s12891-017-1563-8
Ruscitti P, Cipriani P, Masedu F, Romano S, Berardicurti O, Liakouli V et al (2017) Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS ONE 2:e0170108. https://doi.org/10.1371/journal.pone.0170108
Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R (2015) Di Minno MND Subclinical atherosclerosis in patients with rheumatoid arthritis. Thromb Haemost 113(05):916–930. https://doi.org/10.1160/TH14-11-0921
Nurmohamed MT, Heslinga M, Kitas GD (2015) Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 11:693–704. https://doi.org/10.1038/nrrheum.2015.112
Gabriel SE, Crowson CS (2012) (2012) Risk factors for cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol 24:171–176. https://doi.org/10.1097/BOR.0b013e32834ff2fd
Di Minno MND, Iervolino S, Lupoli R, Russolillo A, Coppola A, Peluso R et al (2012) Cardiovascular risk in rheumatic patients: the link between inflammation and atherothrombosis. Semin Thromb Hemost 38:498–505. https://doi.org/10.1055/s-0032-1306433
Chung CP, Avalos I, Raggi P, Stein CM (2007) Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol 26:1228–1233. https://doi.org/10.1007/s10067-007-0548-7
Dessein PH, Joffe BI (2006) When is a patient with rheumatoid arthritis at risk for cardiovascular disease? J Rheumatol 33:201–203
El-Gazzar I, El-Dakrony A-H, Sayed S, El-Fishawy H, Fathi H, Shaaban M et al (2017) Clinical significance of metabolic syndrome and carotid intima-media thickness in Behҫet’s disease patients: Relation to disease activity. Egypt Rheumatol 39:171–174. https://doi.org/10.1016/j.ejr.2016.11.001
Colombo BM, Murdaca G, Caiti M, Rodriguez G, Grassia L, Rossi E et al (2007) Intima-media thickness: a marker of accelerated atherosclerosis in women with systemic lupus erythematosus. Ann N Y Acad Sci 1108:121–126. https://doi.org/10.1196/annals.1422.014
Demir K, Avcı A, Ergulu Esmen S, Tuncez A, Yalcın MU, Yılmaz A et al (2021) Assessment of arterial stiffness and epicardial adipose tissue thickness in predicting the subclinical atherosclerosis in patients with ankylosing spondylitis. Clin Exp Hypertens 43:169–174. https://doi.org/10.1080/10641963.2020.1833025
Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G (2013) Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Card 111:73–78. https://doi.org/10.1016/j.amjcard.2012.08.044
Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H et al (2003) Human epicardial adipose tissue is a source of inflammatory mediators. Circ 108:2460–2466. https://doi.org/10.1161/01.CIR.0000099542.57313.C5
Verhagen SN, Visseren FL (2011) Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis 214:3–10. https://doi.org/10.1016/j.atherosclerosis.2010.05.034
Shemirani H, Meysam KM (2012) Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease-an observational study. Anatol J Cardiol 12:200–205. https://doi.org/10.5152/akd.2012.061
Hwang IC, Park HE, Choi SY (2016) Epicardial adipose tissue contributes to the development of non-calcified coronary plaque: a 5-year computed tomography follow-up study. J Atheroscler Thromb 24:262–274. https://doi.org/10.5551/jat.36467
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III et al (2010) Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthrit Rheum 62:2569–2581. https://doi.org/10.1002/art.27584
Smolen J, Breedveld F, Schiff M, Kalden J, Emery P, Eberl G et al (2003) A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatol 42:244–257. https://doi.org/10.1093/rheumatology/keg072
Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K et al (2005) Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 7:1–11. https://doi.org/10.1186/ar1740
Prevoo M, Van’T Hof MA, Kuper H, Van Leeuwen M, Van De Putte L, Van Riel P (1995) Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthrit Rheum 38:44–48
Nishimura RA, Miller FA Jr, Callahan MJ, Benassi RC, Seward JB, Tajik AJ et al (1985) Doppler echocardiography: theory, instrumentation, technique, and application. Mayo Clin Proc 60:321–343
Tajik AJ, Seward J, Hagler D, Mair D, Lie J (1978) Two-dimensional real-time ultrasonic imaging of the heart and great vessels. Technique, image orientation, structure identification, and validation. Mayo Clin Proc 53:271–303
Sahn DJ, DeMaria A, Kisslo J, Weyman AF (1978) Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58:1072–1083. https://doi.org/10.1161/01.CIR.58.6.1072
Jeong J-W, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK et al (2007) Echocardiographic epicardial fat thickness and coronary artery disease. J Circ 71:536–539. https://doi.org/10.1253/circj.71.536
Chaowalit N, Somers VK, Pellikka PA, Rihal CS, Lopez-Jimenez F (2006) Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis 186:354–359. https://doi.org/10.1016/j.atherosclerosis.2005.08.004
Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A et al (2003) Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocr 88:5163–5168. https://doi.org/10.1210/jc.2003-030698
Iacobellis G, Bianco AC (2011) Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Met 22:450–457. https://doi.org/10.1016/j.tem.2011.07.003
Packer M (2018) Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 71:2360–2372. https://doi.org/10.1016/j.jacc.2018.03.509
Xu Y, Cheng X, Hong K, Huang C, Wan L (2012) How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis 23:227–233. https://doi.org/10.1097/MCA.0b013e328351ab2c
Ding J, Hsu F-C, Harris TB, Liu Y, Kritchevsky SB, Szklo M et al (2009) The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 90:499–504. https://doi.org/10.3945/ajcn.2008.27358
Temiz A, Gökmen F, Gazi E, Akbal A, Barutçu A, Bekler A et al (2015) Epicardial adipose tissue thickness, flow-mediated dilatation of the brachial artery, and carotid intima–media thickness. Herz 40:217–224. https://doi.org/10.1007/s00059-014-4140-z
Fatma E, Bunyamin K, Savas S, Mehmet U, Selma Y, Ismail B et al (2015) Epicardial fat thickness in patients with rheumatoid arthritis. Afr Health Sci 15:489–495. https://doi.org/10.4314/ahs.v15i2.23
Lima-Martínez MM, Campo E, Salazar J, Paoli M, Maldonado I, Acosta C et al (2014) Epicardial fat thickness as cardiovascular risk factor and therapeutic target in patients with rheumatoid arthritis treated with biological and nonbiological therapies. Arthritis. https://doi.org/10.1155/2014/782850
Başpınar N, Solak O, İsmail Ş, Sezer F (2019) Examination of the epicardial fat area in patients with rheumatoid arthritis by computed tomography of the thorax. Cumhur Medical J 41:506–515. https://doi.org/10.7197/223.vi.613537
Ormseth MJ, Lipson A, Alexopoulos N, Hartlage GR, Oeser AM, Bian A et al (2013) Association of epicardial adipose tissue with cardiometabolic risk and metabolic syndrome in patients with rheumatoid arthritis. Arthritis Care Res 65:1410–1415. https://doi.org/10.1002/acr.22027
Karadag DT, Sahin T, Tekeoglu S, Isik OO, Yazici A, Cefle A (2019) Epicardial adipose tissue thickness in systemic sclerosis patients without overt cardiac disease. Rheumatol Int 39:1191–1200. https://doi.org/10.1007/s00296-019-04306-8
Lipson A, Alexopoulos N, Hartlage GR, Arepalli C, Oeser A, Bian A et al (2012) Epicardial adipose tissue is increased in patients with systemic lupus erythematosus. Atherosclerosis 223:389–393. https://doi.org/10.1016/j.atherosclerosis.2012.06.006
Resorlu H, Akbal A, Resorlu M, Gokmen F, Ates C, Uysal F et al (2015) Epicardial adipose tissue thickness in patients with ankylosing spondylitis. Clin Rheumatol 34:295–299. https://doi.org/10.1007/s10067-014-2568-4
Ozdil K, Caliskan Z, Keles N, Ozturk O, Tekin AS, Kahraman R et al (2017) Echocardiographic epicardial fat thickness measurement: A new screening test for subclinic atherosclerosis in patients with inflammatory bowel diseases. North Clin Istanb 4:4–12. https://doi.org/10.14744/nci.2017.74508
Kitterer D, Lotus J, Birkmeier S, Backes M, Braun N, Sechtem U et al (2015) Impact of long-term steroid therapy on epicardial and pericardial fat deposition: a cardiac MRI study. Cardiovasc Diabetol 14:130–140
Karpouzas GA, Rezaeian P, Ormseth SR, Hollan I, Budoff MJ (2021) Epicardial adipose tissue volume is a marker of subclinical coronary atherosclerosis in Rheumatoid arthritis. Arthritis Rheum 73:1412–1420. https://doi.org/10.1002/art.41693
Alpaydın S, Buyukterzi Z, Akkurt HE, Yılmaz H (2017) Impaired left ventricular diastolic functions and thickened epicardial adipose tissue in rheumatoid arthritis patients is correlated with DAS-28 score. Acta Cardiologica Sinica 33:182–187. https://doi.org/10.6515/ACS20160608B
Bacaksız A, Tasal A, Sevgili E, Erdoğan E, Onsun N, Sönmez O et al (2014) Epicardial fat thickness in patients with psoriasis vulgaris. Turk Kardiyol Dern Ars 42(1):47–54. https://doi.org/10.5543/tkda.2014.78949
Baysal E, Yilmaz N, Karadag O, Yaylak B, Altintas B, Altindag R et al (2015) Epicardial adipose tissue thickness and systemic sclerosis. Acta Medica Maditerranea 31:97–101
Petra CV, Albu A, Pamfil C, Tamas MM, Vesa SC, Rednic S (2019) The relationship between epicardial adipose tissue and arterial stiffness in patients with rheumatoid arthritis. Med Ultrason. 21:427–434. https://doi.org/10.11152/mu-2001
Tanindi A, Erkan A, Ekici B (2015) Epicardial adipose tissue thickness can be used to predict major adverse cardiovascular events. Coron Artery Dis 26(8):686–691. https://doi.org/10.1097/MCA.0000000000000296
Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K et al (2013) Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population the heinz nixdorf recall study. Am Col Cardiol 13(61):1388–1395. https://doi.org/10.1016/j.jacc.2012.11.062
Eisenberg E, McElhinney PA, Commandeur F, Chen X, Cadet S, Goeller M et al (2020) Deep learning-based quantification of epicardial adipose tissue volume and attenuation predicts major adverse cardiovascular events in asymptomatic subjects. Circ Cardiovasc Imaging 13:e009829. https://doi.org/10.1161/CIRCIMAGING.119.009829
Albuquerque FN, Somers VK, Blume G, Miranda W, Korenfeld Y, Calvin AD et al (2012) Usefulness of epicardial adipose tissue as predictor of cardiovascular events in patients with coronary artery disease. Am J Cardiol 110:1100–1105. https://doi.org/10.1016/j.amjcard.2012.06.003
Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U et al (2003) Epicardial fat from echocardiography:a new method for visceral adipose tissue prediction. Obes Res 11:304–310. https://doi.org/10.1038/oby.2003.45
Acknowledgements
None to declare
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Ethics approval
TC Sağlık Bakanlığı Sağlık Bilimleri Üniversitesi Dışkapı Yıldırım Beyazıt Eğitim ve Araştırma Hastanesi – Klinik Araştırmalar Etik Kurulu – 19/4/2021- Number: 109/37.
Statements on human and animal rights
All procedures were approved by the Dışkapı Yıldırım Beyazıt Eğitim ve Araştırma Hastanesi Ethical Board.
Informed consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keleşoğlu Dinçer, A.B., Şahan, H.F. Increased epicardial adipose tissue thickness as a sign of subclinical atherosclerosis in patients with rheumatoid arthritis and ıts relationship with disease activity ındices. Intern Emerg Med 19, 1015–1024 (2024). https://doi.org/10.1007/s11739-024-03542-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-024-03542-6