Abstract
Frailty increases vulnerability to adverse outcomes. Long-term conditions increase the risk of frailty. We searched PubMed, Web of Science, The Cochrane Library, EMBASE from inception to March 2022. Quality assessment was conducted using the NOS. Data was analysed in a pooled a random-effects meta-analysis. Our primary outcome was the impact of frailty on mortality in adults with Chronic Obstructive Pulmonary Disease (COPD) diagnosis according to the guidelines. Secondary outcomes were: frailty and association with readmissions, hospitalisations, exacerbation rates, and prevalence of frailty in COPD. We identified 25 studies, with 5882 participants. The median prevalence of frailty was 47% (IQR, 39.3–66.3%, range 6.4–72%). There was an association between COPD patients living with frailty and increased risk of mortality versus COPD patients without frailty (pooled OR, 4.21 (95% CI 2.99–5.93, I2 55%). A descriptive analysis of relationship between frailty and hospital readmission and all cause hospitalization showed positive associations. The relationship between frailty and the risk of exacerbation showed a pooled OR, 1.45 (95% CI 0.37–5.70, I2 80%). Frailty is significantly associated with higher mortality risk in COPD. Frailty is common in patients with COPD and its measurement should be considered in clinical practice to better characterise COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is one of the leading causes of mortality, morbidity, and health-care use worldwide (www.goldcopd.org). It is both preventable and treatable. COPD is one of the most common chronic diseases in old age. COPD diagnosis should be considered in any individual who has chronic dyspnea and cough and a history of exposure to risk factors such as smoking and must be functionally confirmed by spirometry. The disease is frequently associated with chronic comorbidities, including cardiovascular and metabolic disease, that may potentially influence health status and mortality of COPD patients. The prevalence of COPD increases with age and the highest rate is among those > 60 years. COPD prevalence data varies widely across countries which is likely due to different diagnostic criteria. The global prevalence of COPD according to the GOLD definition was 10.3% among people ages 30 to 79 years in 2019 [1]. The lowest estimates of prevalence are based on self-reported diagnosis, is under recognized and often misdiagnosed [2], these numbers underestimate the true prevalence. The burden of COPD is expected to increase over the next decades due to continued exposure to risk factors and ageing of the world’s population. The health care costs associated with COPD are high. In Europe, the direct costs of respiratory disease account for about 6% of health budgets and more than 50% of this is due to COPD. The disease contributes to significant health care burden annually in terms of visits, access to emergency departments and hospitalisations (www.goldcopd.org).
Frailty is a syndrome in which multiple factors reduce physiological capacity and increase an individual’s vulnerability to adverse health outcomes following minor stressor events [3, 4]. People living with frailty are at higher risk of falls, disability, prolonged hospitalisation, admission to care homes, and death [3, 4]. There are two main classifications of frailty (the deficit model and the phenotype model) and many clinical assessment tools used to measure it [4,5,6]. Some instruments use scoring systems and standardised cut-offs based on multiple domains including cognitive and social items, while others use a single functional measurement, such as hand grip strength [6,7,8]. Prevalence of frailty varies according to the criteria model used and the setting in which a population is studied [9].
Chronic diseases, such as lung diseases, are known risk factors for the development of frailty [6, 9]. Not surprisingly frailty is common in people with COPD. The prevalence of frailty in the COPD population varied from 9 to 64% according to the criteria of the phenotype model and from 9 to 28% in studies based on different frailty models (Marengoni et al.) [10]. The literature on frailty in COPD is evolving and frailty in COPD is common in older patients both in primary and secondary care settings [11]. The systematic review and meta-analysis on the relationship between COPD and frailty, published by Marengoni et al. in 2018 [10], demonstrated a two-fold increase of being frail if one has COPD, compared to people without COPD. These data did not explore a link between adverse outcomes associated with frailty and people living with COPD.
The identification of outcomes in patients with COPD living with frailty is important to predict disease progression and improve clinical outcomes [12, 13]. The primary outcomes of this review were to assess the prevalence of frailty in a population with COPD and to determine the association between frailty and mortality in people with COPD. The secondary outcomes were to explore the association between frailty and readmissions, hospitalisations, and exacerbations.
Study design and methods
The systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Metanalyses (PRISMA) recommendations. The protocol was registered through the PROSPERO database (registration number: CRD42022328511).
Search strategy
The search strategy was developed in partnership with a specialist librarian. Two researchers (AV, JL) independently searched four electronic databases (PubMed, Web of Science, The Cochrane Library and EMBASE) for manuscripts published from inception to 24th March 2022. The search terms were based on Medical Subject Headings (MeSH) and the following words referring to frailty and COPD were used as keywords: Pulmonary Disease, Chronic Obstructive Bronchitis, Emphysema, AND frailty. The search strategy is outlined in Supplementary Information (SI) file. Studies reporting information on frailty assessment and COPD in titles and abstracts were included. Any disagreement on study eligibility was resolved through discussion with a third reviewer (JH). A hand search of the reference lists of all relevant articles was performed to identify any articles not captured by the electronic search. Only full-text reports were considered reported in English.
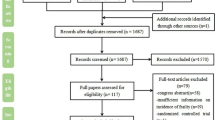
The inclusion criteria were: (1) Study participants (≥ 18 years) who were diagnosed with COPD and were assessed for frailty. We accepted a diagnosis of COPD according to the recognised international GOLD guidelines (www.goldcopd.org) confirmed by spirometry (post-bronchodilator FEV1/FVC < 0.70). Studies with confirmed diagnosis of COPD according to the International Classification of Diseases (ICD-Codes; www.who.int/standards/classifications/classification-of-diseases) were also included; (2) Only studies using a validated method of frailty identification were included; (3) Outcome measures were: prevalence of frailty and related- mortality and morbidity in patients with COPD; (4) Cross-sectional, longitudinal, prospective or retrospective cohort and case–control study designs. The review process is summarised in a PRISMA flow diagram (Fig. 1). Exclusion criteria were patients with COPD listed for lung transplantation.
Data extraction and quality assessment
Study characteristics, demographic information, frailty tool, frailty prevalence and outcomes data were independently extracted from the included studies. Study authors were contacted to clarify or provide additional data where it was missing or unclear.
For the studies included the quality assessment was conducted by the two reviewers independently (AV, JL) and arbitrated by a third (JH) using the Newcastle–Ottawa Scale (NOS) [14], which assesses the risk of bias in observational studies. Each domain examined was classified as good, fair or poor. Studies were considered to be of good quality where they scored good for all domains, fair if they scored fair in one or more domain and poor if they scored poorly in any domain.
Outcomes
The primary outcome was mortality in people with COPD and frailty. A priori, long-term mortality was defined as ≥ 1-year mortality, and short-term mortality was defined as in-hospital mortality or 30- or 90-day mortality after admission for acute exacerbation of COPD. Secondary outcomes were: readmissions to hospital within 30 days or 90 days following hospitalisation for AECOPD; hospitalisations due to any-cause or COPD; and COPD exacerbations rates (patients experiencing at least one COPD exacerbation).
Key exposure of frailty
Studies were included if they measured frailty using any validated instrument (this could be either a deficit index or clinical judgement approach). The following were examples of those included: Fried Frailty Phenotype [4], Frailty Index [5], Clinical Frailty Scale (CFS) [8], FRAIL Scale [15], Canadian Study of Health and Aging Clinical Frailty Scale [16], Reported Edmonton Frailty Scale [17], Kihon Checklist [18], FiND questionnaire [19], PRISMA-7 questionnaire [20], Tilburg Frailty Indicator [21], and Frailty Staging System [22]. Frailty was measured as a binary variable as frail or not frail using the thresholds presented for the individual instruments. For example, within the CFS 4–9 were scored as frail compared to 1–3 that were not frail. Studies using Gait Speed as a surrogate indicator of physical frailty and Time Up and Go Test to assess risk of falls were excluded. We summarised each of frailty instruments in SI-Table 1D.
Data analysis
The prevalence of frailty was estimated as the median study level prevalence, presented alongside the interquartile range.
The primary outcome of mortality was associated with frailty. Only studies that were clinically and contextually homogenous were considered for pooling. Homogenous studies were pooled using a Mantel–Haenszel method with a random-effects. Pooled effects were presented as odds ratio (OR) with associated 95% CIs, p-values, and I2 summary data. All pooled meta-analyses were performed using Review Manager Version 5.4.
Secondary outcomes were narratively described and, where study characteristics were deemed as contextually homogeneous, they were associated with frailty as a binary variable. Where possible secondary outcomes were pooled in a manner consistent with the primary outcome.
Assessment of subgroups and statistical heterogeneity
Statistical heterogeneity was measured using the I2 statistic. Heterogeneity exceeding 80% was explored using subgroup analyses. Pre-specified subgroups to explore heterogeneity included; age; gender; study design, type of frailty instrument, and study level risk of bias.
Results
Search results and quality assessment
After removal of duplicates, 699 records were identified. One hundred and thirteen (113) full texts were reviewed, and 88 of these were excluded for the following reasons: not original research articles (editorial, commentary, ongoing studies not published, review, inconsistent reporting, guideline or congress abstracts); not appropriate study design; duplicate data from same study population; articles on lung transplantation; diagnosis not according to the guidelines. Twenty-five (25) studies included are shown in the PRISMA flowchart (Fig. 1). Eleven studies were determined as good quality [23,24,25,26,27,28,29,30,31,32,33], fourteen were categorised as fair quality [34,35,36,37,38,39,40,41,42,43,44,45,46,47]. For further details of the quality assessments tool, see SI-Table 1A, SI- Table 1B, and SI- Table 1C.
Characteristics of the included studies
The included studies were published between 2018 and 2022, and of the 25 studies, 11 were cohort studies and 14 were cross-sectional (Table 1). The studies originated from Japan [34,35,36, 40], Taiwan [41], China [28, 45], Singapore [32], Turkey [47], Greece [33], Italy [26, 43, 46], Spain [38, 39], the United Kingdom [24, 30], the Netherlands [42], Brazil [23], USA [25, 29, 31], and Canada [27, 44]. Of the 25 studies, 5882 participants were included, 46.5% were male (2735/5882). There was a range of frailty assessment tools used in the included studies, of which 22 were deemed suitable for inclusion in the frailty prevalence estimation. The average age of the participants was 70 years old; this does not include the study by Mustafaoğlu et al. [47] as the mean age was not recorded, although the study reported age range 65–84 (Table 1).
Frailty prevalence in COPD
Prevalence was assessed using 11 different frailty scales, with the most common being the Fried Frailty Phenotype, the Frailty Index, and the Kihon Checklist. Two of the 25 included studies [30, 41] did not report data on prevalence of frailty in COPD. Of 29 included studies, the median prevalence of frailty in COPD patients was 47 (IQR, 39.3–66.3; range 6.4–72%) (SI- Table 1D and SI- Table 1E). The overall prevalence of pre-frailty ranged between 19.6 and 73.7%. Only one study, Gephine et al. [44], included patients with COPD with chronic respiratory failure defined as use of long-term oxygen therapy and/or non-invasive ventilation. The overall prevalence of frailty in COPD was 43% (SI- Table 1C).
Mortality
Seven longitudinal studies explored the relationship between frailty and long-term mortality across 2560 participants [51]. Five studies [28, 29, 32, 50, 52] found a positive association, whilst two studies [25, 26] reported no association.
Two studies [27, 45] explored the influence of frailty on short-term mortality across 544 patients. Both studies reported an association between being frail and mortality.
Overall, there was an association found with patients with COPD living with frailty and increased risk of mortality compared to patients with COPD without frailty pooled OR, 4.21 (95% CI 2.99–5.93, I2 55%) (Fig. 2).
One study, Kennedy et al. [29], conducted a retrospective analysis of 2-year survival data from the 5-year, multicentre study. They found a significantly reduced survival in frail participants with a mortality rate of 36% compared to 16% in non-frail or pre-frail participants. Therefore, we performed a subgroup analysis of the six studies which reported long-term mortality only [25, 26, 28, 32, 50, 52], excluding the data of the Kennedy study [29]. The results showed OR, 0.16 (95% CI 0.07–0.25, I2 84%) (SI-Fig. 3).
Readmission
Three studies [30, 31, 48] investigated readmissions within 30 and 90 days. Of three studies involving 255 patients, all of them [30, 31, 48] found positive associations between frailty and readmission. Witt et al. [31] demonstrated an Odds Ratio of 19.31 (1.07–349.03) in a small sample (n = 70) of which all 8 readmitted patients were living with frailty. Alqahtani and colleagues [30] demonstrated that people who were readmitted had a higher frailty index than those who were not admitted but did not provide data on the exact numbers of people readmitted. Bernabeu-Mora and colleagues [48] reported a trend (p = 0.002) showing increased readmission with increasing frailty [49].
Hospitalisation
Three studies [25, 28, 29] investigated hospitalisations, which included 1491 participants in stable condition. All of these studies reported at some evidence of an association between frailty and hospitalisation. Yee and colleagues [25] demonstrated that frailty phenotype was associated only with non-COPD-related hospitalisations (Incidence Rate Ratio 2.62, 95%CI 1.00–6.84, p = 0.05). Luo et al. [28] showed that all-cause hospitalisations were significantly higher in the frail group versus the non-frail group (p < 0.001). The study of Kennedy et al. [29] found that frailty phenotype was associated with increased incidence of hospitalisations (p = 0.02).
Exacerbations
Three studies [25, 32] explored the relationship between frailty and risk of future moderate-to-severe exacerbations within the next year. Two studies, including 1751 patients, showed no association. One study [28] found a positive association (n = 309) showing that the risk of moderate-to-severe acute exacerbation within one year was higher in patients with COPD and frailty compared to patients without frailty (p < 0.001).
Of the included studies, eight [23, 24, 26, 28, 33, 38, 44] across 1697 participants investigated the relationship between frailty and the number of COPD exacerbations in the past year. Only three studies [28, 38, 44] reported the exact number of patients with ≥ 1 or ≥ 2 exacerbations of COPD within the last year. Only one [28] of these three studies found that, in 309 patients with COPD, people living with frailty had more previous exacerbations than those living without frailty (p < 0.001). Overall, there was no association found with patients with COPD living with frailty and increased risk of previous exacerbations compared to patients with COPD without frailty (pooled OR, 1.45 95%CI 0.37–5.70, I2 80%) (SI-Fig. 4).
Discussion
The main objective of this review was mortality in people with COPD and frailty. The secondary aims were prevalence of frailty in COPD and influence of frailty on hospitalisations, readmissions, and exacerbations in patients with COPD. This study identified 25 studies with 5882 patients. Eleven studies were good quality, and the remaining fourteen fair quality.
This is the first study to systematically review the literature on mortality and frailty in patients with COPD. We demonstrated that all-cause mortality in COPD was associated with being frail in both the short and longer term. Most existing evidence on long-term mortality in COPD includes studies that assess patients hospitalised for AECOPD, both in general medicine wards and Intensive Care Units [53,54,55,56]. The systematic review of Singanayagam et al. [57] concluded that short-term mortality, including in-hospital mortality, was influenced by multiple factors in hospitalised patients for AECOPD such as age and comorbidities which could be inferred as similar to the syndrome of frailty.
The study demonstrated that nearly 50% of patients with COPD, diagnosed using the GOLD criteria, were living with frailty. The frailty instruments used in these studies (Fried model and Frailty Index) [4, 5] are have been extensively validated in people aged over 65 years [58]. In younger populations, they have been used although much less widely [59], therefore generalizing to younger populations with COPD, should be done with caution.
For the prevalence estimate, we included three studies [42,43,44] that explored the relationship between frailty and pulmonary rehabilitation in patients with COPD. Finamore et al. [43] confirmed the influence of frailty on the walking distance during and after the programme and showed greater improvement in rehabilitation outcomes in frail patients compared to non-frail patients [60]. These findings are in line with the study of Maddocks et al. [12]. At the start of rehabilitation programme, ter Beek et al. [42] found high coexistence of malnutrition and frailty in participants with COPD. Gephine et al. [44] reported a greater use of nutritional supplements in patients with COPD with chronic respiratory failure and frailty. Nutritional status is one of the components of assessment in pulmonary rehabilitation and is important in determining frailty. Rehabilitation programmes that improve levels of physical activity and malnutrition can increase quality of life and reduce number of hospitalisations and mortality in patients with COPD [www.goldcopd.org, 60]. These programmes should be recommended to make lifestyle changes that might potentially decrease or reverse frailty in COPD. While not the focus of this systematic review, these studies highlight the interventions to ameliorate the high prevalence of frailty in this population can be beneficial. This emphasizes the need for pulmonary rehabilitation and frailty be studied further in people living with COPD.
The study showed there is some evidence of positive association between the frailty phenotype and hospitalization in patients with COPD. Also, the review found that readmissions to hospital within three months after acute exacerbation were more frequent in patients with COPD and frailty. Although these findings require future studies and larger samples to explore better these relationships, these results are consistent with frailty adversely contributes to a range of poor outcomes in COPD, which would be in line with the literature.
The study investigated the role of frailty and the risk of exacerbations of COPD, with no convincing associations demonstrated. However, only a few studies were identified, confirming the need for further longitudinal reports in this population. Specifically, only a small number of studies reported the exact number of patients who had an exacerbation and the exact number of those exacerbations. Most authors reported the mean or median value of exacerbations making meta-analyses difficult and we would urge future authors to report these detailed data.
A strength of this review is the use of GOLD guidelines for the diagnosis of COPD. These guidelines are the internationally recommended standard for the diagnosis and management of COPD. COPD is commonly self-reported, which is known to underestimate the true prevalence of disease. Therefore, this review provides by far the most accurate estimate of frailty with COPD. This systematic review had some limitations. First, the review included only nonrandomized studies and reverse causality cannot be ignored. Also, while we only considered recognised frailty tools for the diagnosis of frailty, we considered 11 different frailty instruments. Hence some heterogeneity cannot be excluded. However, whilst the different frailty tools may have offered the contextual diversity, they were not able to explain the heterogeneity. This represents an additional weakness of the review. In addition, the length of follow up in the included studies may have explained some of the heterogeneity found in the analysis of long-term mortality.
Future research should explore frailty as a modifiable risk factor and the development of clinical interventions to reverse the effect of frailty such as pulmonary rehabilitation that may, potentially, reduce health-care use and rate of admissions in routine practice.
Conclusion
The study shows a high prevalence of frailty in people with COPD diagnosed according to GOLD criteria. Our review suggests that frailty has a clear association with mortality in COPD. This data can be used to support shared decision-making in hospital settings. Our findings highlight the need of early identification of patients with COPD living with frailty to minimise their risk. Further work is urgently needed to identify a single frailty assessment tool that includes physical, cognitive and social domains for patients with COPD to accurately capture the complexity of the condition.
Data sharing
All data sharing and collaboration requests should be directed to the corresponding author. The data underlying this article are available in the article and in its online Supplementary Information file.
References
Adeloye D, Song P, Zhu Y et al (2022) Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 10(5):447–458
Verduri A, Hewitt J, Carter B, et al (2022) Prevalence of asthma and COPD in a cohort of patients at the follow up after COVID-19 pneumonia. Pulmonology 10:S2531–0437(22)00127–1
Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. Lancet 381(9868):752–762
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–M156
Mitnitski AB, Mogilner AJ, Rockwood K (2001) Accumulation of deficits as a proxy measure of aging. Sci World J 1:323–336
Morley JE, Vellas B, van Kan GA et al (2013) Frailty consensus: a call to action. J Am Med Dir Assoc 14(6):392–397
Aguayo GA, Donneau AF, Vaillant MT et al (2017) Agreement between 35 published frailty scores in the general population. Am J Epidemiol 186(4):420–434
Rockwood K, Song X, MacKnight C et al (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173(5):489–495
Buta BJ, Walston JD, Godino JG et al (2016) Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 26:53–61
Marengoni A, Vetrano DL, Manes-Gravina E et al (2018) The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest 154(1):21–40
Mirza S, Benzo R (2017) Chronic obstructive pulmonary disease phenotypes: implications for care. Mayo Clin Proc 92(7):1104–1112
Maddocks M, Kon SS, Canavan JL et al (2016) Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 71(11):988–995
Bone AE, Hepgul N, Kon S et al (2017) Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis 14(1):85–99
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Abellan van Kan G, Rolland YM, Morley JE et al (2008) Frailty: toward a clinical definition. J Am Med Dir Assoc 9(2):71–72
Chan DC, Tsou HH, Chen CY et al (2010) Validation of the Chinese-Canadian study of health and aging clinical frailty scale (CSHA-CFS) telephone version. Arch Gerontol Geriatr 50(3):e74–e80
Rolfson DB, Majumdar SR, Tsuyuki RT et al (2006) Validity and reliability of the Edmonton frail scale. Age Ageing 35(5):526–529
Ogawa K, Fujiwara Y, Yoshida H et al (2011) The validity of the “Kihon Check-list” as an index of frailty and its biomarkers and inflammatory markers in elderly people. Nihon Ronen Igakkai Zasshi 48(5):545–552
Cesari M, Demougeot L, Boccalon H et al (2014) A self-reported screening tool for detecting community-dwelling older persons with frailty syndrome in the absence of mobility disability: the FiND questionnaire. PLoS One 9(7):e101745
Raiche M, Hebert R, Dubois MF (2008) PRISMA-7: a case-finding tool to identify older adults with moderate to severe disabilities. Arch Gerontol Geriatr 47(1):9–18
Gobbens RJ, van Assen MA, Luijkx KG et al (2010) The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 11(5):344–355
Cacciatore F, Abete P, Mazzella F et al (2005) Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 35(12):723–730
Dias LS, Ferreira ACG, da Silva Junior JLR et al (2020) Prevalence of frailty and evaluation of associated variables among COPD patients. Int J Chron Obstruct Pulmon Dis 15:1349–1356
Gale NS, Albarrati AM, Munnery MM et al (2018) Frailty: A global measure of the multisystem impact of COPD. Chron Respir Dis 15(4):347–355
Yee N, Locke ER, Pike KC et al (2020) Frailty in chronic obstructive pulmonary disease and risk of exacerbations and hospitalizations. Int J Chron Obstruct Pulmon Dis 15:1967–1976
Scarlata S, Finamore P, Laudisio A et al (2021) Association between frailty index, lung function, and major clinical determinants in chronic obstructive pulmonary disease. Aging Clin Exp Res 33(8):2165–2173
Warwick M, Fernando SM, Aaron SD et al (2021) Outcomes and resource utilization among patients admitted to the intensive care unit following acute exacerbation of chronic obstructive pulmonary disease. J Intensive Care Med 36(9):1091–1097
Luo J, Zhang D, Tang W et al (2021) Impact of frailty on the risk of exacerbations and all-cause mortality in elderly patients with stable chronic obstructive pulmonary disease. Clin Interv Aging 16:593–601
Kennedy CC, Novotny PJ, LeBrasseur NK et al (2019) Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc 16(2):217–224
Alqahtani JS, Aldabayan YS, Aldhahir AM et al (2021) Predictors of 30- and 90-Day COPD exacerbation readmission: a prospective Cohort study. Int J Chron Obstruct Pulmon Dis 16:2769–2781
Witt LJ, Spacht WA, Carey KA et al (2021) Weak handgrip at index admission for acute exacerbation of COPD predicts all-cause 30-day readmission. Front Med (Lausanne) 8:611989
Lee SY, Nyunt MSZ, Gao Q et al (2022) Co-occurrence of physical frailty and COPD and association with disability and mortality: Singapore longitudinal ageing study. Chest 161(5):1225–1238
Ierodiakonou D, Kampouraki M, Poulonirakis I et al (2019) Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm Med 19(1):63
Oishi K, Matsunaga K, Harada M et al (2020) A new dyspnea evaluation system focusing on patients’ perceptions of dyspnea and their living disabilities: The linkage between COPD and Frailty. J Clin Med 9(11):3580
Takahashi S, Hirano T, Yasuda K et al (2021) Impact of frailty on hippocampal volume in patients with chronic obstructive pulmonary disease. Biomedicines 9(9):1103
Nishimura K, Nakayasu K, Mori M et al (2021) Are fatigue and pain overlooked in subjects with stable chronic obstructive pulmonary disease? Diagnostics (Basel) 11(11):2029
Kagiali S, Inal-Ince D, Cakmak A et al (2022) Daily living activities, exercise capacity, cognition, and balance in COPD patients with and without frailty. Ir J Med Sci 191(2):817–824
Medina-Mirapeix F, Bernabeu-Mora R, Gimenez-Gimenez LM et al (2018) Physical frailty characteristics have a differential impact on symptoms as measured by the CAT score: an observational study. Health Qual Life Outcomes 16(1):140
Naval E, Gonzalez MC, Giraldos S et al (2021) Frailty assessment in a stable COPD cohort: is there a COPD-frail phenotype? COPD 18(5):525–532
Hirai K, Tanaka A, Homma T et al (2019) Comparison of three frailty models and a sarcopenia model in elderly patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int 19(9):896–901
Chen PJ, Yang KY, Perng WC et al (2018) Effect of dyspnea on frailty stages and related factors in Taiwanese men with COPD. Int J Chron Obstruct Pulmon Dis 13:2463–2469
ter Beek L, van der Vaart H, Wempe JB et al (2020) Coexistence of malnutrition, frailty, physical frailty and disability in patients with COPD starting a pulmonary rehabilitation program. Clin Nutr 39(8):2557–2563
Finamore P, Scarlata S, Delussu AS et al (2021) Frailty impact during and after pulmonary rehabilitation. COPD 18(5):518–524
Gephine S, Mucci P, Grosbois JM et al (2021) Physical frailty in COPD Patients with chronic respiratory failure. Int J Chron Obstruct Pulmon Dis 16:1381–1392
Gu JJ, Liu Q, Zheng LJ (2021) A frailty assessment tool to predict in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 16:1093–1100
Castellana F, Lampignano L, Bortone I et al (2020) Physical frailty, multimorbidity, and all-cause mortality in an older population from southern italy: results from the Salus in Apulia Study. J Am Med Dir Assoc 22(3):598–605
Mustafaoğlu BT, Gulen ST, Birtekokak F et al (2020) Factors affecting frailty syndrome in elderly chronic obstructive pulmonaary disease patients and its relationship with systemic inflammation. Turk Geriatri Dergisi 23(4):446–454
Bernabeu-Mora R, Garcia-Guillamon G, Valera-Novella E et al (2017) Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis 11(10):383–392
Kusunose M, Oga T, Nakamura S et al (2017) Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Resp Res 4:e000196
Galizia G, Cacciatore F, Testa G et al (2011) Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res 23:118–125
Limpawattana P, Putraveephong S, Inthasuwan P et al (2017) Frailty syndrome in ambulatory patients with COPD. Int J Chron Obstruct Pulmon Dis 12:1193–1198
Lahousse L, Ziere G, Verlinden VJA et al (2016) Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci 71(5):689–695
Suissa S, Dell’Aniello S, Ernst P (2012) Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 67(11):957–963
Garcia-Sanz MT, Canive-Gomez JC, Senin-Rial L et al (2017) One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis 9(3):636–645
Gudmundsson G, Ulrik CS, Gislason T et al (2012) Long-term survival in patients hospitalized for chronic obstructive pulmonary disease: a prospective observational study in the Nordic countries. Int J Chron Obstruct Pulmon Dis 7:571–576
Owusuaa C, Dijkland SA, Nieboer D et al (2022) Predictors of mortality in chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med 22(1):125
Singanayagam S, Schembri S, Chalmers JD (2013) Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 10(2):81–89
Hewitt J, Carter B, Vilches-Moraga A et al (2020) The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 5(8):e444–e451
Hanlon P, Lewsey J, Quint JK et al (2022) Frailty in COPD: an analysis of prevalence and clinical impact using UK Biobank. BMJ Open Respir Res 9(1):e001314
Garcia-Aymerich J, Lange P, Benet M et al (2006) Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 61(9):772–778
Support statement
AV was supported by a research fellowship at Cardiff University (UK) funded by CHIESI Italy. The sponsor had no role in study design, analysis, interpretation, or writing of the manuscript. This work has been presented in the form of abstract at the European Geriatric Medicine Society International Congress 2022 in London (UK).
Funding
There was no direct funding was received for this study. This paper represents independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care (BC).
Author information
Authors and Affiliations
Contributions
AV, JH conceived the study, searched the literature, extracted the data, drafted the manuscript. AV, JL searched the literature, extracted the data, reviewed the manuscript. BC, JH developed the methods, arbitrated differences in data extraction, carried out the analysis, interpreted the analysis, and drafted the manuscript. NM advised on the methods for the study, drafted and reviewed the manuscript. CR, EC reviewed the manuscript. JH is the guarantor of this review.
Corresponding author
Ethics declarations
Conflict of interest
We all declare no competing interests.
Research in context
Evidence before this study: Frailty is common in people with COPD. A previous systematic review on the relationship between COPD and frailty demonstrated a two-fold increase of being frail if one has COPD, compared to people without COPD. These data did not explore a link between adverse outcomes associated with frailty and people living with COPD. Added value of this study: This is the first study to systematically review the literature on mortality and frailty in patients with confirmed diagnosis of COPD. These data demonstrated that all-cause mortality in COPD is associated with being frail in both the short and longer term. Implications of all the available evidence: All-cause mortality in COPD is associated with being frail in both the short and longer term. Frailty assessment should be considered in routine daily practice in all people living with COPD.
Human and Animal Rights statement
This article does not contain any studies with humans or animals conducted by any of the authors.
Informed consent
No informed consent was required for this review paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verduri, A., Carter, B., Laraman, J. et al. Frailty and its influence on mortality and morbidity in COPD: A Systematic Review and Meta-Analysis. Intern Emerg Med 18, 2423–2434 (2023). https://doi.org/10.1007/s11739-023-03405-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03405-6