Abstract
Biological age is increasingly recognized as being more accurate than chronological age in determining chronic health outcomes. This study assessed whether biological age, assessed on intensive care unit (ICU) admission, can predict hospital mortality. This retrospective cohort study, conducted in a tertiary multidisciplinary ICU in Western Australia, used the Levine PhenoAge model to estimate each patient’s biological age (also called PhenoAge). Each patient’s PhenoAge was calibrated to generate a regression residual which was equivalent to biological age unexplained by chronological age in the local context. PhenoAgeAccel was a dichotomized measure of the residuals, and its presence suggested that one was biologically older than the corresponding chronological age. Of the 2950 critically ill adult patients analyzed, 291 died (9.9%) before hospital discharge. Both PhenoAge and its residuals (after regressing on chronological age) had a significantly better ability to differentiate between hospital survivors and non-survivors than chronological age (area under the receiver-operating-characteristic curve 0.648 and 0.654 vs. 0.547 respectively). Being phenotypically older than one’s chronological age was associated with an increased risk of mortality (PhenoAgeAccel hazard ratio [HR] 1.997, 95% confidence interval [CI] 1.568–2.542; p = 0.001) in a dose-related fashion and did not reach a plateau until at least a 20-year gap. This adverse association remained significant (adjusted HR 1.386, 95% CI 1.077–1.784; p = 0.011) after adjusted for severity of acute illness and comorbidities. PhenoAgeAccel was more prevalent among those with pre-existing chronic cardiovascular disease, end-stage renal failure, cirrhosis, immune disease, diabetes mellitus, or those treated with immunosuppressive therapy. Being phenotypically older than one’s chronological age was more common among those with comorbidities, and this was associated with an increased risk of mortality in a dose-related fashion in the critically ill that was not fully explained by comorbidities and severity of acute illness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging population is a major public health problem in many developed countries. How to improve quality of health and life of the aging population is an emerging research paradigm [1]. Chronological age has an important association with the biological functions of all mammals but longevity of each species as well as between different individual organisms within the same species is highly variable [2]. Unlike a car or a machine, different living systems have acquired some in-built strategies to modulate and adapt to cellular breakdowns and mutations, even though these strategies will eventually fail. Different biological and pathogenic mechanisms have been proposed to explain why aging or senescence occurs, and whether it is preventable. Deteriorations in mitochondrial functions or activities, DNA methylation, and spontaneous somatic mutations – including Clonal Hematopoiesis of Indeterminate Potential (CHIP) – are currently believed to be most probable key pathogenic mechanisms [3].
Regardless of the precise mechanisms that lead to cellular and organ senescence, a certain phenotypical manifestation of aging can be observed which appears to be more predictive of a person’s health and even longevity than chronological age. Frailty is a well-recognized phenotypical feature of aging [4]. Recently, advanced computing capability including the use of artificial intelligence has allowed researchers to identify biomarkers of DNA methylation and quantify biological age of individuals. Evidence suggests that phenotypical or biological age of a person is a dynamic ‘epigenetic clock’ – which can be dialled forward with diseases and health events such as surgery, pregnancy, or COVID-19 infection [5], and conversely regressed backward with interventions [6] – as compared to chronological age. A phenotypical or biological age model – the Levine PhenoAge — based on data input from nine commonly used blood tests was published in 2018 and it was subsequently validated to be predictive of 10-year survival, cognitive dysfunction, and diabetes mellitus over and beyond a person’s chronological age, education background, socioeconomic status and smoking habit, including among those who are older than 80 years-old [7,8,9,10]. Furthermore, somatic mutations, such as CHIP, are strongly linked to age acceleration in multiple biological age clocks, including the Levine PhenoAge model [3], suggesting that epigenetic aging may be used to identify a population at high risk for adverse health outcomes and who may be a target for clinical interventions.
If phenotypical or biological age is proven to be better than chronological age in reflecting the health status of a person, it would make sense to use biological age instead of chronological age for risk adjustment in health outcome studies. Currently, the ability of biological age to predict outcomes of critically ill patients has not been assessed and its utility remains uncertain. We hypothesized that biological or phenotypical age was more important than chronological age in determining mortality of the critically ill and being biologically older than one’s chronological age would be associated with worse mortality outcome. In this study, we aimed to compare the ability of biological age to chronological age in predicting hospital mortality of critically ill patients. Specifically, we also aimed to assess whether being phenotypically older than one’s chronological age was more common among those with pre-existing chronic health conditions, and phenotypical age more than chronological age could predict mortality after adjusted for severity of acute illness and comorbidities.
Materials and methods
After obtaining research approvals from the Hospital Quality Improvement Unit and Clinical Information System administrative committee (approval number 49386 on February 28, 2023: Performance of the current risk adjustment models with and without PhenoAge in intensive care patients), de-identified data of critically ill patients admitted to the intensive care unit (ICU) of Fiona Stanley Hospital in Western Australia between June 1, 2015 and June 30, 2021 were retrieved from the Clinical Information System. Procedures were followed in accordance with the ethical and data protection standards of the approving committees and with the Helsinki Declaration of 1975. Fiona Stanley Hospital is a tertiary teaching hospital, and its 40-bed ICU admits critically ill patients of most medical and surgical specialties including heart and lung transplantation and burns.
In this retrospective cohort study, we used the Levine’s PhenoAge model to estimate the phenotypical or biological age of each patient [9, 10]. The Levine PhenoAge model was derived using prospectively collected clinical data of the third National Health and Nutrition Examination Survey (NHANES) III [9] and uses biomarkers that reflect DNA methylation which is believed to be one of the key pathogenic processes of aging. It utilizes chronological age as well as nine blood tests are needed to calculate the PhenoAge of the patients. The accuracy of this model was validated using the NHANES IV data (n = 14,008) [10]. The nine blood tests used in the Levine PhenoAge model include C-reactive protein, glucose concentration, mean red blood cell volume, red blood cell distribution width, albumin concentration, creatinine concentration, lymphocyte percentage, alkaline phosphatase, and white cell count. The equations needed to compute PhenoAge are described in Online supplement Table 1.
In addition to calculating the PhenoAge, we also assessed the predictive ability of three PhenoAge-related age measures, including (a) the absolute difference between PhenoAge and chronological age, (b) the residuals from regressing PhenoAge on chronological age using the whole cohort of patients and (c) PhenoAgeAccel – when the residual is positive [9]. The scientific rationale for assessing the latter two PhenoAge related measures are as follows. By including chronological age as a predictor, PhenoAge tends to diverge positively from chronological age as the latter is increasing; using residuals from regressing PhenoAge on chronological age would avoid this potential problem. Another distinct advantage of using the residuals from the regression instead of the absolute difference between PhenoAge and chronological age is that the residuals are indexed to other patients within the same context or study, like a concept called re-calibration based on local context. Most, if not all, existing ICU prognostic models have not incorporated this feature in a standard fashion.
PhenoAgeAccel is a dichotomized representation of the residuals of the regression, patients who have a positive residual signify their phenotypical or biological age years are older than their corresponding chronological age in comparison to all patients included in the study. The distribution of the residuals of the regression relationship between chronological age and PhenoAge of the current study patients, and a scatter plot showing how chronological age was related to PhenoAge for each patient are described in Online supplement Fig. 1a and b, respectively. The constant of the regression line represents the recalibrated constant of the relationship between PhenoAge and the chronological age in the current study context. As such, PhenoAgeAccel after indexed to the rest of the patients in this study was considered to exist only for those who had a PhenoAge in excess of 33.4 years (which is the Y-intercept of the regression line) (Online supplement Fig. 1b) above their corresponding chronological age.
Difference in the survival curves between those with and without PhenoAgeAccel*. a Univariable analysis and b After adjusting for all the confounders including chronological age, elective surgery, chronic cardiovascular disease, chronic respiratory disease, end-stage renal failure, cirrhosis, immune disease, immunosuppressive therapy, metastatic cancer, lymphoma, leukemia, AIDS, and diabetes mellitus. *PhenoAgeAccel was present when the PhenoAge was older than chronological age after indexing one’s PhenoAge with the PhenoAge of the whole cohort
In this study, only patients who had the full set of nine blood tests done within 24 h of ICU admission were included. When any of these nine tests were repeated within the first 24 h of ICU admission, the one closest to the ICU admission time was used for consistency purposes.
Statistical analysis
Descriptive statistics were used to describe the characteristics of the cohort, and categorical and continuous data with skewed distributions were analyzed by Chi Square and Mann–Whitney tests, respectively. Area under the receiver-operating-characteristic curve (AUROC) was first used to assess the discriminative ability of chronological age, PhenoAge and its related measures.
The association between PhenoAgeAccel and hospital mortality was then assessed by a stepwise forward Cox proportional hazards regression to examine the confounding effect of pre-existing chronic health conditions and severity of acute illness (using the Acute Physiology and Chronic Health Evaluation II score). Finally, we used a 4-knot restricted cubic spline function to assess whether residuals of phenotypical age more than chronological age could have a non-linear or plateauing effect on survival time of our patients [11].
Sample size calculation showed that 814 patients would give us 80% statistical power to confirm phenotypical age as a continuous predictor of mortality with an AUROC > 0.6, assuming the overall mortality rate of the cohort was 9% and a two-tailed p-value < 0.05 was taken as significant. To detect an AUROC > 0.65, only 363 patients would be needed. All data were analyzed by SPSS for Windows (version 24. IBM, USA), S-Plus (version 8.2, 2010, Insightful Corp., Seattle, Washington, USA), and MedCalc (version 20.218, 2023).
Results
Of the 17,917 patients admitted to the ICU during the study period, 3175 admissions (17.7%) had the full set of blood tests to calculate patients’ ICU admission PhenoAge (Online supplement Fig. 2). These latter patients were slightly sicker compared to those without the full set of nine blood tests, with a lower proportion of patients who had undergone elective surgery (27.4% vs. 37.5%) with a slightly higher APACHE II score (15.0 vs 14.9) and hospital mortality (9.9% vs. 8.7%).
After excluding ICU readmissions during the same hospitalization (n = 225), 2950 patients were further analyzed in this study. As expected, emergency admissions, chronic respiratory and cardiovascular diseases, cirrhosis, immunosuppressive therapy, lymphoma, AIDS, and a high APACHE II score (of > 15) were more common among those who died after admission (Table 1). Chronological age was also significantly different between survivors and non-survivors (p = 0.008), so were PhenoAge and its related measures. However, the ability of the PhenoAge (AUROC 0.648) and its related measures were all statistically better than chronological age (AUROC 0.547) in differentiating between survivors and non-survivors (Online supplement Fig. 3).
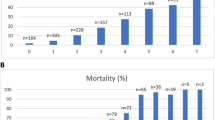
Stepwise forward Cox regression showed that PhenoAgeAccel was significantly associated with an increased risk of mortality (hazard ratio [HR] 1.997, 95% confidence interval [CI] 1.568–2.542; p = 0.001) (Table 2) (Fig. 1a); and this adverse association remained significant (adjusted HR 1.386, 95% CI 1.077–1.784; p = 0.011) (Fig. 1b) after adjusting for multiple pre-existing medical conditions and severity of acute illness. The hazard associated with excessive phenotypical age compared to chronological age was ‘dose-related’ and this effect did not reach a plateau until one’s biological age was close to 20 years older than their chronological age (Fig. 2).
Finally, analysis of the association between PhenoAgeAccel and comorbidities showed that PhenoAgeAccel was indeed more prevalent among those with severe pre-existing chronic cardiovascular disease, end-stage renal failure, cirrhosis, immune disease, diabetes mellitus, or those treated with immunosuppressive therapy (Table 3).
Discussion
This study showed that being phenotypically older than one’s chronological age was common among those with severe pre-existing medical conditions, and this was associated with an increased risk of mortality that was not fully captured or explained by comorbidities and severity of acute illness. These results have some clinical and research implications and require further discussion.
First, clinicians have long held an intuition that biological or phenotypical age is more important than chronological age in determining health outcomes, and chronological age is an imperfect marker of one’s physiological reserve or ‘effective age’ [10, 12]. With intensive computing capability including the use of artificial intelligence, a few phenotypical or biological age estimation models that are predictive of long-term functional and survival outcomes in a wide range of clinical settings have been developed [7,8,9,10, 13,14,15,16]. The utility of biological age in acute care setting has, however, not been thoroughly assessed. Our results confirmed the clinicians’ intuition that phenotypical age was more predictive of mortality than chronological age, even though on its own PhenoAge was still far less predictive of mortality compared to the APACHE II model. Nonetheless, the adverse effect of being biological older than expected did appear to add predictive value to severity of acute illness and comorbidities in predicting mortality. The reasons why PhenoAgeAccel is potentially useful in addition to the APACHE II model might be because PhenoAgeAccel is indexed to the rest of patients included in the study within the same context, such that any significant changes in the blood chemistry used to assess PhenoAgeAccel were calibrated in reference to other critically ill patients in the same critical care setting, making it less likely to be affected by acute inflammatory response and acute resuscitation such as fluid therapy. For this reason, PhenoAgeAccel did not exist in our patients until their PhenoAge was at least 33.4 years older than their corresponding chronological age (Online supplement Figure 1a). Furthermore, diabetes mellitus [17], C-reactive protein [18], lymphocyte count, red blood cell width distribution [19, 20], as well as mean red blood cell volume [21], are not included as predictors in the APACHE II model. If our results can be confirmed by other centers, use of phenotypical age instead of chronological age as a risk adjustment tool for clinical audits and research on critically ill patients should be seriously considered.
Second, our results showed that the difference between PhenoAge and chronological age after indexed to the local context had a relatively linear ‘dose-related’ relationship to hospital mortality that did not reach a plateau until up to a 20-year gap. Emerging studies have demonstrated that phenotypical age is more like a dynamic clock [5]; this would also mean that phenotypical age is like a quantitative assessment of physiological reserve, and there is an opportunity for interventions to ‘dial back’ the phenotypical age of critically ill patients, like non-critically ill patients [22, 23]. A recent study also showed that Clonal Hematopoiesis of Indeterminate Potential (CHIP) had a direct correlation with biological age, including the PhenoAge [3], providing strong biological rationale to support the prognostic significance of PhenoAge. We argue that it is scientifically valid to use phenotypical age as one of the secondary outcomes when assessing effectiveness of medical interventions in phase II clinical trials involving critically ill patients.
Third, we showed that PhenoAgeAccel was associated with a number of comorbidities. This result was not surprising because PhenoAge estimation requires some blood parameters (e.g. blood glucose and alkaline phosphatase) that are also often abnormal in some comorbidities (such as diabetes mellitus and cirrhosis, respectively). Medical advances have prolonged lifespan of many individuals who have serious chronic illnesses including HIV infection [24]. Our next clinical challenge is to improve health span by reducing biological age, and reduce frailty and the burden of chronic health conditions for the aging population [1, 6, 25].
Finally, we need to acknowledge the limitations of this study. Although the sample size of this study was not small, it is a single-center observational study and selection bias is probable. The selected patients represented only a small proportion of all admissions (17.7%) and they were slightly sicker than those who did not have the full set of nine blood tests to allow us to estimate their PhenoAge. Confirmation of the external validity our results by a prospective multicenter study is, therefore, needed. As we alluded to earlier, serial measurements of phenotypical age will be a useful addition in any prospective studies because it would help us to understand how phenotypical age would change during critical illness (or a lack of it), and whether interventions, such as intensive nutritional support or physical rehabilitation, could improve it [6, 25]. Recent studies suggested that biological age is likely related to frailty in the elderly [26], and frailty could be even more important than markers of DNA methylation in predicting long-term health outcomes [4]. Whether biological age remains important as a prognostic factor after accounting for one’s frailty in the critically care setting remains unknown, and we plan to investigate this important issue in our next study. The Levine PhenoAge model belongs to the second-generation DNA methylation age algorithm [27]. A number of other biological age models including the third-generation DNA methylation age algorithms have been published since [13]; whether one model is superior to the other biological age models (e.g. GrimAge model) and also Clonal Hematopoiesis of Indeterminate Potential (CHIP) in predicting mortality of the critically ill has not been investigated [3, 28, 29], but this requires further study.
In conclusion, this proof of concept study showed that being phenotypically older than one’s chronological age after indexed to the local study context – also called PhenoAgeAccel – was common among those with severe comorbidities. The divergence between biological and chronological age had a relatively linear ‘dose-related’ relationship with the risk of hospital mortality in the critically ill that was not fully explained or captured by comorbidities and severity of acute illness. Further studies are needed to assess whether medical interventions could reduce biological age [25], ultimately improving patient-centered outcomes.
Data availability
It will be available for reasonable requests.
References
Partridge L, Slagboom Deelen J, PE. (2018) Facing up to the global challenges of ageing. Nature 56:45–56. https://doi.org/10.1038/s41586-018-0457-8
Austad SN (2010) Methusaleh’s Zoo: how nature provides us with clues for extending human health span. J Comp Pathol 142(Suppl 1):S10-21. https://doi.org/10.1016/j.jcpa.2009.10.024
Nachun D, Lu AT, Bick AG, Natarajan P, Weinstock J, Szeto MD, Kathiresan S, Abecasis G, Taylor KD, Guo X, Tracy R, Durda P, Liu Y, Johnson C, Rich SS, Van Den Berg D, Laurie C, Blackwell T, Papanicolaou GJ, Correa A, Raffield LM, Johnson AD, Murabito J, Manson JE, Desai P, Kooperberg C, Assimes TL, Levy D, Rotter JI, Reiner AP, Whitsel EA, Wilson JG, Horvath S, Jaiswal S, NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium (2021) Clonal hematopoiesis associated with epigenetic aging and clinical outcomes. Aging Cell 20(6):e13366. https://doi.org/10.1111/acel.13366
Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM (2017) The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience 39(1):83–92. https://doi.org/10.1007/s11357-017-9960-3
Poganik JR, Zhang B, Baht GS, Tyshkovskiy A, Deik A, Kerepesi C, Yim SH, Lu AT, Haghani A, Gong T, Hedman AM, Andolf E, Pershagen G, Almqvist C, Clish CB, Horvath S, White JP, Gladyshev VN (2023) Biological age is increased by stress and restored upon recovery. Cell Metab 35(5):807-820.e5. https://doi.org/10.1016/j.cmet.2023.03.015
Zhang B, Trapp A, Kerepesi C, Gladyshev VN (2022) Emerging rejuvenation strategies-Reducing the biological age. Aging Cell 21(1):e13538. https://doi.org/10.1111/acel.13538
Faul JD, Kim JK, Levine ME, Thyagarajan B, Weir DR, Crimmins EM (2023) Epigenetic-based age acceleration in a representative sample of older Americans: Associations with aging-related morbidity and mortality. Proc Natl Acad Sci USA 120(9):e2215840120. https://doi.org/10.1073/pnas.2215840120
Schmitz LL, Zhao W, Ratliff SM, Goodwin J, Miao J, Lu Q, Guo X, Taylor KD, Ding J, Liu Y, Levine M, Smith JA (2022) The socioeconomic gradient in epigenetic ageing clocks: evidence from the multi-ethnic study of atherosclerosis and the health and retirement study. Epigenetics 17(6):589–611. https://doi.org/10.1080/15592294.2021.1939479
Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S (2018) An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 10(4):573–591. https://doi.org/10.18632/aging.101414
Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M (2018) A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLoS Med 15(12):e1002718. https://doi.org/10.1371/journal.pmed.1002718
Ho KM, Knuiman M, Finn J, Webb SA (2008) Estimating long-term survival of critically ill patients: the PREDICT model. PLoS One 3(9):e3226. https://doi.org/10.1371/journal.pone.0003226
Spiegelhalter D (2016) How old are you, really? Communicating chronic risk through “effective age” of your body and organs. BMC Med Inform Decis Mak 16:104. https://doi.org/10.1186/s12911-016-0342-z
McCrory C, Fiorito G, Hernandez B, Polidoro S, O’Halloran AM, Hever A, Ni Cheallaigh C, Lu AT, Horvath S, Vineis P, Kenny RA (2021) GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J Gerontol A Biol Sci Med Sci 76(5):741–749. https://doi.org/10.1093/gerona/glaa286
Ma Q, Li BL, Yang L, Zhang M, Feng XX, Li Q, Liu H, Gao YJ, Ma WZ, Shi RJ, Xue YB, Zheng XP, Gao K, Mu JJ (2022) Association between phenotypic age and mortality in patients with multivessel coronary artery disease. Dis Markers 2022:4524032. https://doi.org/10.1155/2022/4524032
Ma Z, Zhu C, Wang H, Ji M, Huang Y, Wei X, Zhang J, Wang Y, Yin R, Dai J, Xu L, Ma H, Hu Z, Jin G, Zhu M, Shen H (2023) Association between biological aging and lung cancer risk: cohort study and Mendelian randomization analysis. iScience 26(3):106018. https://doi.org/10.1016/j.isci.2023.106018
Nannini DR, Cortese R, Egwom P, Palaniyandi S, Hildebrandt GC (2023) Time to relapse in chronic lymphocytic leukemia and DNA-methylation-based biological age. Clin Epigenetics 15(1):81. https://doi.org/10.1186/s13148-023-01496-8
Pari B, Gallucci M, Ghigo A, Brizzi MF (2023) Insight on infections in diabetic setting. Biomedicines 11(3):971. https://doi.org/10.3390/biomedicines11030971
Ho KM, Lee KY, Dobb GJ, Webb SA (2008) C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: a prospective cohort study. Intensive Care Med 34(3):481–487. https://doi.org/10.1007/s00134-007-0928-0
Wang ZH, Fu BQ, Lin YW, Wei XB, Geng H, Guo WX, Yuan HQ, Liao YW, Qin TH, Li F, Wang SH (2022) Red blood cell distribution width: A severity indicator in patients with COVID-19. J Med Virol 94(5):2133–2138. https://doi.org/10.1002/jmv.27602
Said AS, Spinella PC, Hartman ME, Steffen KM, Jackups R, Holubkov R, Wallendorf M, Doctor A (2017) RBC distribution width: biomarker for red cell dysfunction and critical illness outcome? Pediatr Crit Care Med 18(2):134–142. https://doi.org/10.1097/PCC.0000000000001017
Bateman RM, Sharpe MD, Singer M, Ellis CG (2017) The effect of sepsis on the erythrocyte. Int J Mol Sci 18(9):1932. https://doi.org/10.3390/ijms18091932
Lohman T, Bains G, Cole S, Gharibvand L, Berk L, Lohman E (2023) High-Intensity interval training reduces transcriptomic age: a randomized controlled trial. Aging Cell 20:e13841. https://doi.org/10.1111/acel.13841
Fitzgerald KN, Campbell T, Makarem S, Hodges R (2023) Potential reversal of biological age in women following an 8-week methylation-supportive diet and lifestyle program: a case series. Aging (Albany NY) 15:1833–1839. https://doi.org/10.18632/aging.204602
Marcus JL, Leyden WA, Alexeeff SE, Anderson AN, Hechter RC, Hu H, Lam JO, Towner WJ, Yuan Q, Horberg MA, Silverberg MJ (2020) Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open 3(6):e207954. https://doi.org/10.1001/jamanetworkopen.2020.7954
Singh P, Gollapalli K, Mangiola S, Schranner D, Yusuf MA, Chamoli M, Shi SL, Lopes Bastos B, Nair T, Riermeier A, Vayndorf EM, Wu JZ, Nilakhe A, Nguyen CQ, Muir M, Kiflezghi MG, Foulger A, Junker A, Devine J, Sharan K, Chinta SJ, Rajput S, Rane A, Baumert P, Schönfelder M, Iavarone F, di Lorenzo G, Kumari S, Gupta A, Sarkar R, Khyriem C, Chawla AS, Sharma A, Sarper N, Chattopadhyay N, Biswal BK, Settembre C, Nagarajan P, Targoff KL, Picard M, Gupta S, Velagapudi V, Papenfuss AT, Kaya A, Ferreira MG, Kennedy BK, Andersen JK, Lithgow GJ, Ali AM, Mukhopadhyay A, Palotie A, Kastenmüller G, Kaeberlein M, Wackerhage H, Pal B, Yadav VK (2023) Taurine deficiency as a driver of aging. Science 380(6649):eabn9257. https://doi.org/10.1126/science.abn9257
Ji L, Jazwinski SM, Kim S (2021) Frailty and biological age. Ann Geriatr Med Res 25(3):141–149
Sugden K, Caspi A, Elliott ML, Bourassa KJ, Chamarti K, Corcoran DL, Hariri AR, Houts RM, Kothari M, Kritchevsky S, Kuchel GA, Mill JS, Williams BS, Belsky DW, Moffitt TE (2022) Alzheimer’s disease neuroimaging initiative*. Association of pace of aging measured by blood-based DNA methylation with age-related cognitive impairment and dementia. Neurology 99:e1402–e1413. https://doi.org/10.1212/WNL.0000000000200898
Lu AT, Binder AM, Zhang J, Yan Q, Reiner AP, Cox SR, Corley J, Harris SE, Kuo PL, Moore AZ, Bandinelli S, Stewart JD, Wang C, Hamlat EJ, Epel ES, Schwartz JD, Whitsel EA, Correa A, Ferrucci L, Marioni RE, Horvath S (2022) DNA methylation GrimAge version 2. Aging (Albany NY) 14(23):9484–9549. https://doi.org/10.18632/aging.204434
Jaiswal S, Libby P (2020) Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol 17(3):137–144. https://doi.org/10.1038/s41569-019-0247-5
Acknowledgements
We would like to thank the Fiona Stanley Hospital intensive care unit Clinical Information System (CIS) team, including Hayley Robinson, Sonya Trewren, Sarah Hunter, Miriam Pawar, and Sara Stanley for their assistance in retrieving the data from CIS; without their help, the completion of this study would not be possible.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was solely funded internally by the Department of Intensive Care Medicine, Fiona Stanley Hospital, Perth, WA 6150.
Author information
Authors and Affiliations
Contributions
KMH: concept of the study, design, data analysis, interpretation of the results, and drafting of the manuscript. DJM: data analysis, interpretation of the results. MJ: data extraction and interpretation. CE: infrastructure support, data interpretation and drafting of the manuscript. All authors agreed with the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare in relation to the subject matter and material discussed in this manuscript.
Ethics approval and consent to participate
Research approvals from the Hospital Quality Improvement Unit and Clinical Information System administrative committee were obtained (approval number 49386 on February 28, 2023: Performance of the current risk adjustment models with and without PhenoAge in intensive care patients) in using completely de-identified data of critically ill patients admitted to the intensive care unit (ICU) of Fiona Stanley Hospital in Western Australia between June 1, 2015 and June 30, 2021 from the Clinical Information System. Procedures were followed in accordance with the ethical and data protection standards of the approving committees and with the Helsinki Declaration of 1975.
Human and animal rights, informed consent
Informed consent was waived due to use of de-identified retrospective data and the large number of patients included in the study including many who had died during the hospitalization or after discharge since their first admission.
Consent for publication
Waiver of consent for using de-identified administrative data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ho, K.M., Morgan, D.J., Johnstone, M. et al. Biological age is superior to chronological age in predicting hospital mortality of the critically ill. Intern Emerg Med 18, 2019–2028 (2023). https://doi.org/10.1007/s11739-023-03397-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03397-3