Abstract
Background
An association with aortic aneurysm has been reported among patients with atrial fibrillation (AF). The aims of this study were to investigate the prevalence of thoracic aorta aneurysm (TAA) among patients with AF and to assess whether the co-presence of TAA is associated with a higher risk of adverse clinical outcomes.
Methods and results
Using TriNetX, a global federated health research network of anonymised electronic medical records, all adult patients with AF, were categorised into two groups based on the presence of AF and TAA or AF alone. Between 1 January 2017 and 1 January 2019, 874,212 people aged ≥ 18 years with AF were identified. Of these 17,806 (2.04%) had a TAA. After propensity score matching (PSM), 17,805 patients were included in each of the two cohorts. During the 3 years of follow-up, 3079 (17.3%) AF patients with TAA and 2772 (15.6%) patients with AF alone, developed an ischemic stroke or transient ischemic attack (TIA). The risk of ischemic stroke/TIA was significantly higher in patients with AF and TAA (HR 1.09, 95% CI 1.04–1.15; log-rank p value < 0.001)
The risk of major bleeding was higher in patients with AF and TAA (OR 1.07, 95% CI 1.01–1.14), but not significant in time-dependent analysis (HR 1.04, 95% CI 0.98–1.10; log-rank p value = 0.187),
Conclusion
This retrospective analysis reports a clinical concomitance of the two medical conditions, and shows in a PSM analysis an increased risk of ischemic events in patients affected by TAA and AF compared to AF alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A thoracic aortic aneurysm (TAA) is a localized dilatation of the ascending and thoracic aorta that can lead to dissection and rupture of the vessel wall [1, 2]. The aortic aneurysm may silently progress with one in two cases being completely asymptomatic [1, 2], and indeed diagnosed incidentally during imaging studies performed for other clinical conditions. The first clinical manifestation may occur as an acute event, either aortic dissection, which associates with a high-risk for mortality or cardiovascular event [1].
Recent observational studies have reported a high prevalence of aortic aneurysms among patients with AF, which is the most common cardiac arrhythmia worldwide [3, 4]. However, the clinical significance of concomitant AF and aortic aneurysms remains undetermined. More specifically, the added risk for cerebrovascular events by the concomitant presence of aneurysms of the aorta in patients with AF is unknown. Currently, indication to oral anticoagulation (OAC) therapy in patients with AF in most guidelines is based on risk stratification built on the pattern of comorbidities and summarized in clinical risk scores, such as the CHA2DS2-VASc score [5, 6]. In this score, the ‘V’ component has been framed to include myocardial infarction (including significant coronary artery disease on cardiac imaging), peripheral vascular diseases and the presence of atherosclerotic aortic plaque [7]. Nevertheless, diseases of the aorta such as aortic aneurysms are not formally considered in the ‘V’ criterion of the CHA2DS2-VASc score [6].
In this study using a global federated database of electronic health records, amongst patients with AF, the aims were to examine 1) the prevalence of TAA; and 2) associations between TAA and risk of ischemic stroke, systemic thromboembolic events and major bleeding.
Methods
We used TriNetX, a global federated health research network with real-time updates of anonymised electronic medical records (EMRs). The network includes healthcare organisations (HCOs, academic medical centres, specialty physician practices and community hospitals), with data for > 80 million patients predominately based in the United States. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating HCOs and their individual contribution to each dataset are not disclosed. As a federated research network, studies using the TriNetX health research network do not require ethical approval as no patient identifiable information is received.
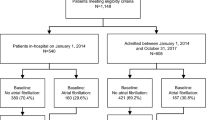
For the present study, the TriNetX research network was searched for the inclusion of AF patients (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] code: I48) aged ≥ 18 years between 1 January 2017 and 1 January 2019. The cohort was placed into two groups based on the presence of AF and TAA with/without rupture (ICD-10-CM codes: I71.1 and I71.2, respectively) or AF alone (Fig. 1). In the TAA group, TAA should to have occurred in the 2017–2019 timeframe, whereas the group with AF alone should not have history ever of TAA. All diagnoses were identified by the ICD-10-CM codes in the EMRs. No other inclusion or exclusion criteria were defined. The searches were run in TriNetX on 22 February 2022. At the time of the search, there were 58 participating HCOs within the TriNetX research network.
Patients have been not involved in the design of the study; however, dissemination of the results is planned thorough patients’ associations.
Follow-up and clinical outcomes
All patients were followed up from inclusion to at least 3-years. The primary endpoint was the occurrence of ischemic stroke/transient ischemic attack (TIA). All-cause mortality, major bleeding (composite of intracranial haemorrhage [ICH] and gastrointestinal bleeding) and the composite of any arterial or venous thrombotic event (any of the following: myocardial infarction, other arterial thrombosis, venous thromboembolism [VTE], or ischemic stroke/TIA, systemic embolism) were secondary outcomes.
Statistical analysis
Continuous variables were expressed as mean and standard deviation (SD), and tested for differences with independent-sample t tests. Categorical variables were expressed as absolute frequencies and percentages, and tested for differences with chi-squared tests.
The TriNetX platform was used to run 1:1 propensity score matching (PSM) using logistic regression. The platform uses ‘greedy nearest-neighbour matching’ with a calliper of 0.1 pooled standard deviations and difference between propensity scores ≤ 0.1. Covariate balance between groups was assessed using standardised mean differences (SMDs). Any baseline characteristic with a SMD between cohorts < 0.1 is considered well matched [8].
Odds ratios (OR) with 95% confidence intervals (CI) were calculated following PSM. Hazard Ratios (HR) and 95% CI were also provided after PSM, as well as Kaplan–Meier survival curves with log-rank tests. No imputations were made for missing data. Two-sided p values < 0.05 were accepted as statistically significant. Statistical analysis was performed using the TriNetX Analytics function in the online research platform.
Results
Participant characteristics
Between January 2017 and 2019, 874,212 patients aged ≥ 18 years with AF were identified. Of these, 17,806 had a TAA with or without rupture accounting for an overall prevalence of 2.04%. Table 1 summarizes the baseline characteristics of patients with AF and TAA and patients without TAA, before and after PSM. Patients with AF and TAA had a higher risk profile with higher prevalence of comorbidities except for diabetes. After PSM, both cohorts were well balanced.
Ischemic stroke/TIA in patients with TAA and AF vs. those with AF alone
After PSM, 17,805 patients were included in each of the two cohorts (i.e. 1:1). During the 3-years of follow-up, 3079 (17.3%) AF patients with TAA and 2772 (15.6%) patients with AF alone developed an ischemic stroke/TIA. The risk of suffering from ischemic stroke/TIA was 1.13-fold higher in patients with AF and TAA (OR 1.13 95% CI 1.07–1.20), confirmed in the time-dependent analysis (HR 1.09, 95% CI 1.04–1.15; log-rank p value < 0.001) (Fig. 2).
Secondary outcomes
After PSM, 6232 (35%) AF patients with TAA and 5872 (33%) patients with AF alone, suffered an arterial or venous thrombotic event during the 3-years of follow-up. For major bleeding, corresponding figures were 2500 (14%) and 2358 (13.2%), respectively; whereas for all-cause mortality they were 3027 (17%) and 3113 (17.5%). Of note, patients with AF and TAA had an increased risk of composite arterial or venous thrombotic events in comparison to patients with AF alone (OR 1.09, 95% CI 1.05–1.14; HR 1.05, 95% CI 1.01–1.09, log-rank p value = 0.004) (Supplementary Fig. 1). Regarding other secondary outcomes, the risk of major bleeding was higher in patients with AF and TAA (OR 1.07, 95% CI 1.01–1.14), but this was not significant in time-dependent analysis (HR 1.04, 95% CI 0.98–1.10; log-rank p value = 0.187). The risk of all-cause mortality was higher in patients with AF alone only in time-dependent analysis (OR 1.03, 95% CI 0.98–1.08; HR 1.06, 95% CI 1.01–1.12, log-rank p value = 0.017).
Discussion
The principal findings of this analysis are as follows: (i) there is a clinical co-occurrence of TAA and AF; (ii) patients with AF and TAA are characterized by a higher cardiovascular risk profile, compared to those with AF and no history of TAA; and (iii) in PSM analysis, amongst patients with AF, TAA was associated with an increased risk of ischemic and systemic thromboembolic events at 3-year follow-up.
Emerging clinical evidence has shown a high prevalence of TAA among patients with AF. In a retrospective analysis from nationwide population database, Hsu et al. [3] reported a bidirectional association between aortic aneurysm and AF, showing that in patients with AF compared to those without AF, an increased incidence of aortic aneurysm was evident at 13 years follow-up (adjusted HR 1.24, 95% CI 1.10–1.40, p < 0.001). Similarly, patients with aortic aneurysm had a higher risk for presenting with AF at follow-up compared to patients without a diagnosis of aortic aneurysm (adjusted HR 1.187, 95% CI 1.079–1.301, p < 0.001) [3]. In a sub-analysis considering only TAA, a higher incidence was detected in patients with AF compared to those without (0.14% vs. 0.09%, p < 0.001) [3].
A cross-sectional study of patients with AF undergoing gated chest computer tomography performed as part of the assessment for pulmonary vein isolation, reported a TAA prevalence of 20%, with 1% of the TAA detected having a size approaching the current surgery indication [4]. From a pathophysiological perspective, atherosclerosis underlies TAA, and indeed, peripheral or coronary artery diseases are other common clinical manifestations of atherosclerosis. Both peripheral or coronary artery diseases are associated with incident AF and AF-related complications, and AF is a common complication after aortic procedures such as trans-catheter aortic valve replacement [9]. The findings regarding AF and TAA could be a non-casual association related to the increasing prevalence of both diseases with advancing age and consequently shared risk factors such as hypertension and heart failure. Indeed, the prevalence of TAA is approximately 4% in patients > 65 years and accounts for 6000 deaths a year in the UK [2]. Similarly, the prevalence of AF increases exponentially with age with an estimated ~ 6.9% prevalence in people > 65 years, though the burden of mortality linked to AF remains more elusive [10].
The finding in this study of a co-occurrence of TAA among patients with AF is aligned with previous results, though our figure being based on EMRs can include also AF post-surgery and therefore be an overestimate. While the associations may simply reflect shared risk factors, these findings raise the clinical implications regarding monitoring of patients with AF for the risk of developing TAA. Of note, the data show a higher prevalence of cardiovascular risk factors in patients with TAA and AF (but not diabetes) which may outline a more hemodynamic and degenerative nature of the TAA than an atherosclerotic origin.
Another major finding of this study is that patients with AF and TAA have an increased risk of stroke, TIA and systemic thromboembolic events compared to a matched AF population with a similar cardiovascular risk profile. Any attempt to provide a plausible mechanistic explanation for such a finding remains speculative although it may be hypothesized that the presence of an aneurysm may be linked to endothelial damage which is one pillar of Virchow’s triad [11]. Also, the presence of complex aortic plaque on the descending aorta is an independent risk factor for ischemic stroke in AF patients [5].
In a general population, the Aortic Plaques and Risk of Ischemic Stroke (APRIS) study [12] and the Stroke Prevention: Assessment of Risk in a Community (SPARC) [13] have recently questioned prior studies [14, 15], reporting a lack of association between the presence of a complex aortic plaque and risk of stroke at a general population level. On the other side, the association between TAA and aortic atherosclerotic plaque remains elusive while it has been shown for involvement of the abdominal and infra-renal aortic aneurysm [16].
In this analysis, there was an increased odd for major bleeding in the group with AF and TAA. The short-term follow-up we considered in this retrospective analysis may have biased this outcome, and the concomitant use of aspirin and/or OAC may have contributed to this. The increased mortality we detected in the group with AF alone compared to patients with AF and TAA which can be counterintuitive due to the well-known mortality linked to TAA, is possibly because patients with TAA of any size not requiring surgery were also included, as our search was based on ICD codes. Therefore, small thoracic aneurysms with slow rates of growth and no impact on survival have been potentially included.
Clinical implications
We believe that the relevance of our finding is linked to the clinical perspective. Though this association seems a discordant comorbidity, AF and TAA have been shown to share commonalities in pathological pathways [17], the requirement of a CT scan to detect diseases of thoracic aorta has limited the applicability of screening program to general population and cannot be supported among AF for the risk benefit ratio considering the overall low figure of AF associated with TAA [17].
The added piece of the results of our analysis is that the coexistence of the two clinical conditions may confer a higher risk of adverse outcomes. Indeed, this claims attention for the need of optimizing the comprehensive medical management which may be difficult to integrate since the two diseases seem different. As a matter of fact, considering the higher cardiovascular risk profile of patients with AF and TAA, the proportion on antiplatelets appears to be high, while OAC is low, being 58.6 and 70.6%, respectively. This finding may be correlated to a perception from the surgeons of a bleeding risk which may lead to hold OAC notwithstanding the increased risk of stroke posed by AF. Similarly, considering the bulk of evidence showing that statins improve outcomes in both AF [18] and TAA [19], the proportion of patients on statins seems to be low. It may be hypothesized that the contradictory findings on the medical therapy prescribed may reflect an absence of an integrated management of both conditions.
Limitations
Several limitations should be considered when interpreting the results of the current study. First, the participant information is based on EMRs, and from this, a distinction between pre-existing AF, new onset of AF and AF post-operative surgery cannot be made. This may explain the low proportion of patients on OAC therapy in the group with only AF, which is difficult to investigate further. Second, patients with thoraco-abdominal aneurysms were excluded in order to assess only the impact of TAA. Thirdly, information on the prevalence of complex aortic plaque in the two groups could not be recovered. In this study, the cohorts were PSM for several factors including age, sex, ethnicity and co-morbidities, but residual confounding factors may still be present and some health conditions may be underreported in EMRs. Finally, the analyses presented in this manuscript have been performed using the TriNetX platform which has the major limitations that data cannot be exported for the analysis. As a result, the graphical output of the software is not optimal and sometimes hinder a proper graphical appreciation of differences that are actually significant.
Conclusion
Our retrospective analysis from a large global federated dataset reports a clinical concomitance of AF and TAA. Importantly, in a PSM analysis, an increased risk of ischemic events in patients affected by both TAA and AF was evident, compared to AF alone. Whether this association simply reflects shared risk factors or commonality in pathophysiological pathways, it raises relevant clinical implications that deserve further investigation.
Data availability
The data that support the findings of this study are available from TriNetX. To gain access to the data, a request can be made to TriNetX (https://live.trinetx.com), but costs may be incurred, and a data sharing agreement is needed.
References
Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS et al (1997) What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 113(3):476–491 (discussion 89-91)
Lopuszko A, Patrick Tan SZC, Munir W, Bashir M (2021) Aortic aneurysm disease-Make room for chronobiology. J Card Surg 36(7):2496–2501
Hsu CC, Chien WC, Wang JC, Chung CH, Liao WI, Lin WS et al (2018) Association between Atrial Fibrillation and Aortic Aneurysms: a population-based cohort study. J Vasc Res 55(5):299–307
Ramchand J, Bansal A, Saeedan MB, Wang TKM, Agarwal R, Kanj M et al (2021) Incidental thoracic aortic dilation on chest computed tomography in patients with atrial fibrillation. Am J Cardiol 140:78–82
Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH et al (2021) Focused update consensus guidelines of the Asia Pacific heart rhythm society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost 122(1):20–47
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al (2021) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42(5):373–498
Zhang J, Lenarczyk R, Marin F, Malaczynska-Rajpold K, Kosiuk J, Doehner W et al (2021) The interpretation of CHA2DS2-VASc score components in clinical practice: a joint survey by the European Heart Rhythm Association (EHRA) Scientific Initiatives Committee, the EHRA Young Electrophysiologists, the association of cardiovascular nursing and allied professionals, and the European Society of Cardiology Council on Stroke. Europace 23(2):314–322
Haukoos JS, Lewis RJ (2015) The propensity score. JAMA 314(15):1637–1638
Yankelson L, Steinvil A, Gershovitz L, Leshem-Rubinow E, Furer A, Viskin S et al (2014) Atrial fibrillation, stroke, and mortality rates after transcatheter aortic valve implantation. Am J Cardiol 114(12):1861–1866
Davis RC, Hobbs FD, Kenkre JE, Roalfe AK, Iles R, Lip GY et al (2012) Prevalence of atrial fibrillation in the general population and in high-risk groups: the ECHOES study. Europace 14(11):1553–1559
Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH (2010) Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin Med Res 8(3–4):168–172
Russo C, Jin Z, Rundek T, Homma S, Sacco RL, Di Tullio MR (2009) Atherosclerotic disease of the proximal aorta and the risk of vascular events in a population-based cohort: the Aortic Plaques and Risk of Ischemic Stroke (APRIS) study. Stroke 40(7):2313–2318
Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O’Fallon WM, Pascoe RD et al (1999) Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study Stroke. Prevention: assessment of risk in a Community. Mayo Clin Proc 74(9):862–869
Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S (2009) Aortic arch plaques and risk of recurrent stroke and death. Circulation 119(17):2376–2382
Kronzon I, Tunick PA (2006) Aortic atherosclerotic disease and stroke. Circulation 114(1):63–75
Kojima K, Kimura S, Hayasaka K, Mizusawa M, Misawa T, Yamakami Y et al (2019) Aortic plaque distribution, and association between aortic plaque and atherosclerotic risk factors: an aortic angioscopy study. J Atheroscler Thromb 26(11):997–1006
Proietti R, Lip GYH, Akhtar R, Field M (2022) Thoracic aortic aneurysms and atrial fibrillation: commonality in pathophysiological pathways. Cardiovasc Res 118(4):e32–e35
Pastori D, Baratta F, Di Rocco A, Farcomeni A, Del Ben M, Angelico F et al (2021) Statin use and mortality in atrial fibrillation: a systematic review and meta-analysis of 100,287 patients. Pharmacol Res 165:105418
Stein LH, Berger J, Tranquilli M, Elefteraides JA (2013) Effect of statin drugs on thoracic aortic aneurysms. Am J Cardiol 112(8):1240–1245
Funding
None.
Author information
Authors and Affiliations
Contributions
RP and JMRC: design of the study, data analysis, and drafting of the manuscript. SLH and BJRB: revision of data analysis and final revision of the manuscript. RLG, FM, TF, JM, RA, PU and MF: critical revision of the manuscript. GHYL: design of the study and critical revision of the manuscript. Guarantor: RP.
Corresponding author
Ethics declarations
Conflict of interest
JMRC: consultant for Idorsia Pharmaceuticals LTD. GYHL: consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. There is nothing to disclose for other authors.
Ethical approval
Not required.
Informed consent
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Proietti, R., Rivera-Caravaca, J.M., Harrison, S.L. et al. Thoracic aortic aneurysm and atrial fibrillation: clinical associations with the risk of stroke from a global federated health network analysis. Intern Emerg Med 18, 423–428 (2023). https://doi.org/10.1007/s11739-022-03184-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-03184-6