Abstract
To assess the efficacy of modified hydration on contrast-associated acute kidney injury (CA-AKI) in ST-segment elevation myocardial infarction (STEMI) after primary percutaneous coronary intervention (pPCI). A total of 438 patients were randomly assigned to 2 groups. The traditional hydration group (group I) was given at a rate of 1 ml/kg/h for 24 h, and the modified hydration group (group II) was given at a rate of 3 ml/kg/h in the first 4 h, and then reduced to 1 ml/kg/h for 12 h. 0.3 mg/kg of furosemide was given 1-h after hydration. The primary endpoint was the incidence of CA-AKI, and the secondary endpoint was the incidence of major adverse cardiovascular events (MACEs) during a median of 22.4 months (IQR 9.6, 32.6 months) follow-up. The incidence of CA-AKI was 8.7%. Among these, Group I was 9.1% and group II was 8.2%, respectively. There was no significant difference in CA-AKI and creatinine levels between the two hydration groups. Multivariable logistics regression analysis revealed that creatinine, white blood cells, and N-terminal pro-B-type natriuretic peptide were associated with CA-AKI. Moreover, CA-AKI was an independent predictor for all-cause death and cardiac death during the follow-up period. The modified hydration may reduce the incidence of CA-AKI, although this difference was not statistically significant. The relationship between CA-AKI and mortality strengthened as creatinine times above baseline increased. Mitigating the occurrence of CA-AKI may reduce all-cause death and cardiac death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widespread adoption of primary percutaneous coronary intervention (pPCI) has significantly improved the cardiovascular outcomes of ST-segment elevation myocardial infarction (STEMI), however, the incidence of contrast-associated acute kidney injury (CA-AKI), which has replaced the definition of contrast-induced acute kidney injury (CI-AKI) in current guidelines [1, 2], may increase due to the emergency of STEMI, attendant hemodynamic compromise and the use of higher contrast volumes [3, 4]. A previous study has shown that the incidence of CA-AKI in patients with STEMI who underwent pPCI occurred in up to 30% [5]. CA-AKI can be preventable in those patients if the underlying risk factors are well identified and managed properly during the acute event [6]. Reducing the amount of contrast media as much as possible and using optimal hydration before and immediately after percutaneous coronary intervention (PCI) are recommended for reducing the incidence [7]. Notably, inadequate hydration markedly increases the incidence of CA-AKI, while excessive hydration may increase the risk of heart failure [8]. Therefore, the identification of optimal hydration dose for patients which can appropriately and effectively prevent CA-AKI is important. But there is no guidance or consensus on how to hydrate in patients undergoing pPCI.
The pharmacokinetics of the contrast agent showed that 80% of the contrast agent was excreted by the kidney in the first 4 h and the remaining 20% was excreted slowly within the next 24 h [8]. The hemodynamic response to intra-arterial injection of contrast agent is biphasic: a brief initial increase in renal blood flow followed by a prolonged decline of 10% to 25% below baseline [9]. Therefore, based on the pharmacokinetics of the contrast agent, we studied the effect of modified hydration in a prospective randomized controlled trial involving patients with STEMI undergoing pPCI.
Methods
Study population
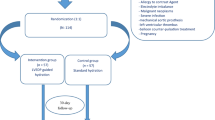
A total of 508 STEMI patients undergoing pPCI at the Cardiovascular Center of Beijing Friendship Hospital from November 2018 to October 2021 were enrolled in this study. 70 patients were excluded according to the exclusion criteria including acute respiratory failure; continuous renal replacement therapy or prior kidney transplantation; uncontrolled congestive heart failure; exposure to contrast agent within 7 days before pPCI or 3 days after pPCI; malignant tumor; patients who did not complete hydration due to various reasons (Supplementary Fig. 1). Finally, consecutive 438 patients were included in the final analysis. All patients were followed up to December 2021 with a median follow-up of 22.4 months (IQR 9.6, 32.6 months).
The local institutional review board at our hospital approved the study protocol, and this study was in accordance with the Declaration of Helsinki.
Study protocol
Eligible patients were randomly assigned to group I (traditional hydration group) and group II (modified hydration group). The traditional hydration group received a continuous intravenous infusion of isotonic saline at a rate of 1 ml/kg/h for 24 h at the beginning of pPCI [10]; the modified hydration group received a continuous intravenous infusion of isotonic saline at a rate of 3 ml/kg/h at the beginning of pPCI for 4 h. After 1 h of hydration, 0.3 mg/kg of furosemide was given for diuretic effect (except patients with systolic blood pressure < 90 mmHg). After 4 h, the rate was reduced to 1 ml/kg/h for 12 h. Equal intravenous hydration volume was given in the two groups. There was no further hydration treatment before and after the study protocol. Computer-generated random numbers determined randomization. All patients were treated with the same nonionic, equal osmolarity iodinated contrast medium during the procedure.
Other data collection and definitions
Venous blood samples were taken from all patients pre-pPCI and then 24, 48 and 72 h after pPCI, samples were used for measurement of serum creatinine and estimated glomerular filtration rate (eGFR) based on Modification of Diet in Renal Disease (MDRD) equation in (ml/min/1.73m2). Patients’ demographic information, medical and medication history, and laboratory measurements were collected and confirmed through electronic medical records. The total ischemic time (the time from symptom initiation to reperfusion) was also collected [11]. The left ventricular ejection fraction (LVEF) was determined using 2-dimensional echocardiography during the index hospitalization.
STEMI was diagnosed based on the diagnostic criteria recommended by the European Society of Cardiology (ESC): (1) chest pain lasting more than 30 min.; (2) the dynamic electrocardiogram change: ST-segment elevation was measured at the J-point at least in two contiguous leads with ST-segment elevation of 2.5 mm in men < 40 years, 2 mm in men 40 years, or 1.5 mm in women in leads V2–V3 and/or 1 mm in the other leads in the absence of left ventricular hypertrophy or left bundle branch block; (3) levels of serum markers of myocardial injury were changed; (4) the coronary angiography showed that patients could receive PCI for anatomy in infarct-related artery[12]. The Killip score class on admission was determined based on chest radiograph findings, lung sounds on auscultation, and blood pressure. In brief, patients were classified into four categories: Killip class I, STEMI without heart failure; Killip class II, STEMI with mild to moderate heart failure (as the presence of rales and/or jugular venous distension); Killip class III, STEMI with pulmonary edema; and Killip class IV, STEMI with cardiogenic shock [13, 14].
Coronary angiography and PCI
Patients with STEMI received coronary angiography immediately after admission to the catheter laboratory, with a digital subtraction machine. The coronary angiography was performed by cardiologists in Beijing Friendship Hospital, and all STEMI patients received PCI surgery as soon as possible after admission to open the infarct-related coronary artery. Meanwhile, two interventional cardiologists judged the stenosis of the coronary artery by a double-blind method.
Study endpoints
The primary endpoint of the study was the incidence of CA-AKI, which identifies events based on Improving Global Outcomes (KDIGO) serum creatinine criteria [increase in serum creatinine by ≥ 26.5 µmol/l (0.3 mg/dl) or increase in serum creatinine by ≥ 1.5 times baseline value]. AKI was said to be present if these changes occurred within 72 h from the initial contrast dose [2, 15]. Stage 1 is characterized by an increase in serum creatinine of > 26.53 μmol/l (0.3 mg/dl) or 1.5 to 1.9 times increase above baseline; stage 2 by 2.0 to 2.9 times increase above baseline; and stage 3 by > 3 times increase above baseline, or an increase of > 353.68 μmol/l (4.0 mg/dl), or initiation of renal replacement therapy.
The secondary endpoint was the occurrence of major adverse cardiac events (MACEs) including all-cause death, cardiac death, non-fatal myocardial infarction (MI), coronary revascularization, and cardiac rehospitalization. All-cause death was defined as the incidence of cardiac death or non-cardiac death. Cardiac death was defined as fatal MI and heart failure, sudden death, and other cardiac death. Non-fatal MI was defined as chest pain with new ST-segment changes and elevation of myocardial necrosis markers to at least twice the upper limit of the normal range. Coronary revascularization was defined as the revascularization of the target vessel or non-target vessels. Cardiac rehospitalization referred to rehospitalization for angina pectoris or heart failure.
Statistical analysis
Depending on the distribution of the data, continuous variables were expressed as mean value ± SD or median and interquartile range (IQR). Frequencies and percentages were used to describe categorical data. Differences between continuous and categorical variables were assessed using Student’s T-test, analysis of variance, Chi-square test, and repeated measures ANOVA as appropriate. Multivariable logistics regression analysis was conducted to analyze the independent risk factors for CA-AKI. Multivariable Cox regression analysis was conducted to analyze the independent risk factors for MACEs in STEMI patients after pPCI. The cumulative incidence of MACEs was estimated by Kaplan–Meier survival curves, and the groups were compared using the log-rank test. Hydration difference on secondary endpoint was assessed with analyses of P for interaction. All analyses were two-tailed and P value < 0.05 was considered statistically significant. Data were analyzed using SPSS statistical package version 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics in traditional and modified hydration group
As shown in Table 1, 438 eligible patients (mean age 60.8 years, men 76.9%) were included in our study, more than half of the patients (60.5%, N = 265) were identified as having hypertension, 28.3% (N = 124) had diabetes mellitus (DM), 5.5% (N = 24) had CHD, 2.3% (N = 10) had a history of MI. Compared with group I, group II showed a significantly higher percentage of current/ex-smokers, higher LVEF, lower percent of triple-vessel lesions, lower SYNTAX score, and lower α1-microglobulin and microalbumin.
Serum creatinine and eGFR in traditional and modified hydration group
As shown in Table 2, CA-AKI occurred in 38 patients, and the incidence was 8.7%, including 20 patients (9.1%) in group I and 18 patients (8.2%) in group II (P = 0.734). Of these, the 32 (7.3%) had stage 1, 4 (0.9%) had stage 2 and 2 (0.5%) had stage 3.
The baseline serum creatinine levels were 73.7 ± 27.5umol/l in group I and 74.1 ± 26.5umol/l in group II. The creatinine levels continuously increased at 24-h, 48-h and 72-h after pPCI both in the two groups. There were significant differences in creatinine at different time points in each hydration group (P < 0.001) (Supplementary Fig. 2A). However, hydration did not affect the serum creatinine levels between the two groups (all P > 0.05) (Table 2).
The baseline eGFR levels were102.4 ± 42.0 ml/min/1.73m2 in group I and 102.2 ± 37.7 ml/min/1.73m2 in group II (P = 0.969). After pPCI, eGFR levels continuously decreased at 24-h, 48-h and 72-h. There were significant differences in eGFR at different time points in each hydration group (P < 0.001) (Supplementary Fig. 2B). However, there was also no significant difference in eGFR between the two groups (all P > 0.05) (Table 2).
Based on KDIGO criteria, we further classified CA-AKI for analysis. Briefly, grade1 was characterized by an increase in serum creatinine of < 1.25 times increase above baseline; grade 2 was 1.25–1.4 times increase above baseline; grand 3 was 1.5–1.9 times increase above baseline; grade 4 was > 2.0 times increase above baseline. As shown in Supplementary Fig. 3, there were no statistical differences in the different grades between the two hydration groups.
Baseline characteristics in CA-AKI and no CA-AKI group, and multivariable logistic regression analysis
Compared with the no CA-AKI group, the CA-AKI group showed a significantly lower percentage of male and current/ex-smokers, higher white blood cells (WBC), hypersensitivity C-reactive protein, and glycosylated hemoglobin, higher peak value of N-terminal pro-B-type natriuretic peptide(pNT-proBNP), peak value of troponin I, and urine α1-microglobulin (Supplementary Table 1).
Multivariable logistic regression analysis was conducted to identify risk factors related to the development of CA-AKI. The results revealed that creatinine (OR = 0.975, 95% CI: 0.952 to 0.999, P = 0.042), WBC (OR = 1.166, 95% CI: 1.034 to 1.315, P = 0.012) and NT-proBNP (OR = 1.013, 95% CI 1.004 to 1.022, P = 0.006) were associated with the incidence of CA-AKI (Table 3).
Kaplan–Meier analysis of secondary endpoint
During a median follow-up period of 22.4 months, the incidence of MACEs was 21.1% in the CA-AKI group and 9.3% in the no CA-AKI group. The incidences of all-cause death and cardiac death were 10.5% and 7.9% in the CA-AKI group, 0.5% and 0.5% respectively in the no CA-AKI group (all P < 0.05). We also compared the secondary endpoint in different hydration groups, there was no statistically significant (Supplementary Table 2). The Kaplan–Meier curves show that the CA-AKI group had a significantly higher cumulative rate of MACEs, all-cause death and cardiac death than the no CA-AKI group (P = 0.006 vs. P < 0.001 vs. P < 0.001 respectively), and all P for interactions > 0.05 (Fig. 1).
Independent association between MACEs, all-cause death and cardiac death
In the multivariable Cox regression analysis, we included variables that were identified to be significantly associated with MACEs, all-cause death and cardiac death in the univariable model. The multivariable Cox regression analysis revealed that CA-AKI was significantly and independently associated with all-cause death and cardiac death. The relationship between CA-AKI and mortality strengthened as creatinine times above baseline increased (Fig. 2). However, after multivariable adjustment, CA-AKI was not associated with MACEs in our study cohort (Table 4 and Fig. 2).
Creatinine grades and risk for development of secondary endpoints. MACEs major adverse cardiac events, CA-AKI contrast-associated acute kidney injury; N-terminal pro-B-type natriuretic peptide. Grade1 was characterized by an increase in serum creatinine of < 1.25 times increase above baseline; grade 2 was 1.25 to 1.4 times increase above baseline; grand 3 was 1.5–1.9 times increase above baseline; grade 4 was > 2.0 times increase above baseline. Model adjusted for sex, age, NT-proBNP, modified hydation and CA-AKI
Discussion
In this study cohort with STEMI patients undergoing pPCI, we found that the patients with lower creatinine were more likely to occur CA-AKI. The incidence of CA-AKI and creatinine levels at different time points were not significantly different between the traditional hydration group and the modified hydration group. CA-AKI have a potential predictive value for all-cause death and cardiac death at the follow-up period. Mitigating the occurrence of CA-AKI in STEMI patients may reduce post-pPCI all-cause death and cardiac death, and improve clinical outcomes.
The incidence of CA-AKI varies greatly after pPCI depending on the baseline demographic, clinical characteristics, diagnostic criteria and angiographic circumstances [5, 16]. Our study showed the incidences of CA-AKI were 8.7%. The main mechanism of CA-AKI includes contrast agent renal tubular toxicity and renal hemodynamic changes resulting in medullary hypoxia stress [9, 17]. Cholesterol micro-embolism also can induce acute renal dysfunction, which was a rare and subtle complication of invasive endovascular procedures. Inflammation may be the mechanism [18]. Hydration can increase blood volume, inhibit the activation of the renin–angiotensin–aldosterone system (RAAS) and the feedback of renal tubules, and dilute the concentration of contrast to prevent renal injury [19]. For our study, it’s the first time to forward new hydration that is based on the biphase of renal hemodynamics and the pharmacokinetics of contrast agent. Compared with the traditional hydration, the modified hydration relatively reduced the incidence of CA-AKI in STEMI patients after pPCI, although there was no statistically different. Trails with large samples are further needed.
The high level of NT-proBNP was responsible for the occurrence of CA-AKI in our study. NT-proBNP is secreted in response to increased volume or pressure overload, or myocardial ischemia [20, 21]. Clinical observations showed patients with heart failure had a decreased renal blood flow as a result of neurohormonal activation and inflammation, increased oxidative stress further compound these pathophysiologic mechanisms [9, 22]. The activation of RAAS and the sympathetic nervous system can further stimulate NT-proBNP[23]. In addition, inflammation may play an important role in the initiation and extension phases of CA-AKI. Inflammatory cells infiltrate the interstitium of the kidney via cytokines produced by renal endothelial cells, and cause the release of oxygen radicals, vasoconstrictors, and thromboxanes, leading to kidney damage [24, 25]. STEMI patients had increased systemic inflammatory responses. Therefore, WBC count was a risk factor for developing CA-AKI. Interestingly, we found lower creatinine levels may more likely to occur CA-AKI in STEMI patients undergoing pPCI. A previous study showed that patients who had contrast material in the course of cardiac catheterization may undergo procedural complications that can affect renal perfusions, such as arrhythmias, MI, and other vascular complications that do not occur to the same degree after vein contrast injections [26]. Therefore, the STEMI patients were particularly likely to have concurrent comorbidities that affect renal function. CA-AKI may be a diagnosis of misattribution or represents a condition obscured by other factors contributing to acute kidney injury. Other conditions caused by the entire range of conditions, treatments, and laboratory variations may also alter creatinine levels. These may affect the association between creatinine and CA-AKI. These results question the utility of creatinine as a marker for CA-AKI. Previous studies observed a reduction in the rise of creatinine after angiography withholding ACEI/ARB [27, 28]. However, a reduction in eGFR and increase in creatinine is known to occur with ACEI/ARB. The contrast medium can increase natriuresis due to its osmotic effect. These results in an activation of the glomerular tubule feedback with vasoconstriction of the afferent arteriole. Concomitant RAAS inhibitor therapy can cause pathological reduction of eGFR [29]. Based on the inconsistent results, we further assessed the significance of RAAS inhibition to CA-AKI, the multivariable result was not statistically significant.
We found that CA-AKI had a predictive value for all-cause death and cardiac death, that consistent with previous studies [12, 30, 31]. The relationship between CA-AKI and mortality strengthened as creatinine times above baseline increased. Greater incidence of procedural cardiac complications, including MI, elevated creatine kinase, hypotension and cardiac arrest in CA-AKI patients may be the reason [32]. In addition, the total ischemic time didn’t show statistics difference between the two hydration groups and between the CA-AKI group and no CA-AKI group, which had been proved to be associated with mortality in patients with STEMI undergoing pPCI [11]. To sum up, although there was no statistical difference, the modified hydration relatively reduced the incidence of CA-AKI and would be a benefit to the prognosis in STEMI patients undergoing pPCI. Therefore, trials with large samples are needed in future research.
Study limitations
Despite the precise randomization, which results in an unimportance difference in baseline characteristics of the two groups, our study has several limitations. First, although our study had a relatively large sample size, the single-center study was also a limitation. Second, although many potential interfering covariables were adjusted by our models, we cannot rule out residual confounding factors or potential selection bias. Third, creatinine as the CA-AKI diagnostic indicator is insensitive, the pre-PCI serum creatinine levels may not be the real baseline serum creatinine level because of possible unstable clinical situations. However, our definition of CA-AKI is dependent on serum creatinine, which is an incomplete marker for kidney function. Fourth, few patients with renal insufficiency were included (56/438), this will affect the modified hydration effect.
Conclusion
According to the findings, although this difference was not statistically significant, the modified hydration also relatively reduced the incidence of CA-AKI after pPCI. CA-AKI may be related to lower creatinine, WBC count and NT-proBNP. The relationship between CA-AKI and mortality strengthened as creatinine times above baseline increased. Therefore, modified hydration may be effective in preventing CA-AKI and improving long-term clinical outcomes. Further studies with a larger sample size are recommended.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MI:
-
Myocardial infarction
- CHD:
-
Coronary heart disease
- OMI:
-
Old myocardial infarction
- WBC:
-
White blood cells
- hsCRP:
-
Hypersensitivity C-reactive protein
- eGFR:
-
Estimated glomerular filtration rate
- HbA1C:
-
Glycosylated hemoglobin
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- LDL-C:
-
Low density lipoprotein cholesterol
- HDL-C:
-
High density lipoprotein cholesterol
- pCKMB:
-
Peak value of creatine kinase isoenzyme-MB
- pTNI:
-
Peak value of troponin I
- pNT-proBNP:
-
Peak value of N-terminal pro-B-type natriuretic peptide
- LVEF:
-
Left ventricular ejection fraction
- LM:
-
Left main trunk
- pPCI:
-
Primary percutaneous coronary intervention
- STEMI:
-
ST-segment elevation myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- MACEs:
-
Major adverse cardiovascular events
- CA-AKI:
-
Contrast-associated acute kidney injury
References
Kodzwa R (2019) ACR manual on contrast media: 2018 updates. Radiol Technol 91:97–100
Davenport MS, Perazella MA, Yee J et al (2020) Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American college of radiology and the national kidney foundation. Radiology 294:660–668
Khalfallah M, Abdalaal M, Adel M (2019) Contrast-Induced nephropathy in patients with ST-segment elevation myocardial infarction: is it affected by treatment strategy? Glob Heart 14:295–302
Khalfallah M, Allaithy A, Maria DA (2021) Incidence, predictors and outcomes of contrast induced nephropathy in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Glob Heart 16:57
Maioli M, Toso A, Leoncini M et al (2011) Effects of hydration in contrast-induced acute kidney injury after primary angioplasty: a randomized, controlled trial. Circ Cardiovasc Interv 4:456–462
Khalfallah M, Abdelmageed R, Allaithy A (2020) Very early versus early percutaneous coronary intervention in patients with decreased e-GFR after successful fibrinolytic therapy. Glob Heart 15:34
Ma G, Li M, Teng W et al (2022) Febuxostat combined with hydration for the prevention of contrast-induced nephropathy in hyperuricemia patients undergoing percutaneous coronary intervention: A CONSORT-compliant randomized controlled trial. Medicine 101:e28683
Liu Y, Li H, Chen S et al (2016) excessively high hydration volume may not be associated with decreased risk of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with renal insufficiency. J Am Heart Assoc 5:e003171
Bansal S, Patel RN (2020) Pathophysiology of contrast-induced acute kidney injury. Interv Cardiol Clin 9:293–298
Cui T, Zhao J, Bei W et al (2017) Association between prophylactic hydration volume and risk of contrast-induced nephropathy after emergent percutaneous coronary intervention. Cardiol J 24:660–670
Coiro S, Cavallini C (2020) Impact of mobile intensive care units on STEMI delays and outcomes-Is it simply a matter of time? Eur J Intern Med 73:27–29
Ibánez B, James S, Agewall S et al (2017) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol 70:1082
El-Menyar A, Zubaid M, AlMahmeed W (2012) Killip classification in patients with acute coronary syndrome: insight from a multicenter registry. Am J Emerg Med 30:97–103
Taguchi E, Konami Y, Inoue M et al (2017) Impact of Killip classification on acute myocardial infarction: data from the SAIKUMA registry. Heart Vessels 32:1439–1447
Van der Molen AJ, Reimer P, Dekkers IA et al (2018) Post-contrast acute kidney injury—part 1: definition, clinical features, incidence, role of contrast medium and risk factors : recommendations for updated ESUR contrast medium safety committee guidelines. Eur Radiol 28:2845–2855
Hua R, Ding N, Guo H (2022) Contrast-induced acute kidney injury in patients on SGLT2 inhibitors undergoing percutaneous coronary interventions: a propensity-matched analysis. Front Cardiovasc Med 9:918167
Cheng W, Zhao F, Tang CY et al (2018) Comparison of iohexol and iodixanol induced nephrotoxicity, mitochondrial damage and mitophagy in a new contrast-induced acute kidney injury rat model. Arch Toxicol 92:2245–2257
Fukumoto Y, Tsutsui H, Tsuchihashi M et al (2003) The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol 42:211–216
Marenzi G, Ferrari C, Marana I et al (2012) Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (induced diuresis with matched hydration compared to standard hydration for contrast induced nephropathy prevention) trial. JACC Cardiovasc Interv 5:90–97
Nadir MA, Witham MD, Szwejkowski BR, Struthers AD (2011) Meta-analysis of B-type natriuretic peptide’s ability to identify stress induced myocardial ischemia. Am J Cardiol 107:662–667
Xia WJ, Huang YY, Chen YL et al (2011) Acute myocardial ischemia directly modulates the expression of brain natriuretic peptide at the transcriptional and translational levels via inflammatory cytokines. Eur J Pharmacol 670:7–12
Schrier RW (2006) Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol 47:1–8
Weinfeld MS, Chertow GM, Stevenson LW (1999) Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am Heart J 138:285–290
Yamamoto Y, Yamamoto N, Kanematsu Y (2020) High white blood cell count is a risk factor for contrast-induced nephropathy following mechanical thrombectomy for acute ischemic stroke. Cerebrovasc Dis Extra 10:59–65
Akcay A, Nguyen Q, Edelstein CL (2009) Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009:137072
McDonald RJ, McDonald JS, Bida JP et al (2013) Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology 267:106–118
Bainey KR, Rahim S, Etherington K et al (2015) Effects of withdrawing vs continuing renin-angiotensin blockers on incidence of acute kidney injury in patients with renal insufficiency undergoing cardiac catheterization: results from the angiotensin converting enzyme inhibitor/angiotensin receptor blocker and contrast induced nephropathy in patients receiving cardiac catheterization (CAPTAIN) trial. Am Heart J 170:110–116
Maccarrone R, Di Lullo L, Forcella M et al (2020) New strategies for prevention and early diagnosis of iodinated contrast-induced nephropathy: a systematic review. G Ital Nefrol 37:2020-S75
Bakris GL, Weir MR (2000) Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 160:685–693
Weisbord SD, Chen H, Stone RA et al (2006) Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol 17:2871–2877
Giacoppo D, Madhavan MV, Baber U et al (2015) Impact of contrast-induced acute kidney injury after percutaneous coronary intervention on short- and long-term outcomes: pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv 8:e002475
Rihal CS, Textor SC, Grill DE et al (2002) Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105:2259–2264
Acknowledgements
The authors gratefully acknowledge the contributions of all staffs who work on the Cardiovascular Center of Beijing Friendship Hospital Data Bank (CBD BANK).
Funding
This study was supported by National Key R&D Program of China (2021ZD0111004), Natural Science Foundation of China (No. 82070357), Beijing Municipal Administration of Hospital Incubating Program (No. PX2018002), Beijing Key Clinical Subject Program, and GE Healthcare 【GE Healthcare (Shanghai) Co. Ltd 】.
Author information
Authors and Affiliations
Contributions
HC and HWL: contributed to the conception or design of the work. LL and LZ: contributed to the acquisition, analysis, or interpretation of data for the work. HC and WPL: contributed discussion and edited manuscript. LL: drafted the manuscript. All authors critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The study data collections were approved by the Institutional Review Board of Beijing Friendship Hospital, Capital Medical University, and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, L., Zhou, L., Li, W. et al. Role of modified hydration for preventing contrast-associated acute kidney injury in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Intern Emerg Med 18, 67–76 (2023). https://doi.org/10.1007/s11739-022-03109-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-03109-3