Abstract
Transient ischemic attack (TIA) is a neurologic emergency characterized by cerebral ischemia eliciting a temporary focal neurological deficit. Many clinical prediction scores have been proposed to assess the risk of stroke after TIA; however, studies on their clinical validity and comparisons among them are scarce. The objective is to compare the accuracy of ABCD2, ABCD2-I, and OTTAWA scores in the prediction of a stroke at 7, 90 days, and 1 year in patients presenting with TIA. Single-centre, retrospective study including patients with TIA admitted to the Emergency Department of our third-level, University Hospital, between 2018 and 2019. Five hundred three patients were included. Thirty-nine (7.7%) had a stroke within 1 year from the TIA: 9 (1.7%) and 24 (4.7%) within 7 and 90 days, respectively. ABCD2, ABCD2-I, and OTTAWA scores were significantly higher in patients who developed a stroke. AUROCs ranged from 0.66 to 0.75, without statistically significant differences at each time-point. Considering the best cut-off of each score, only ABCD2 > 3 showed a sensitivity of 100% only in the prediction of stroke within 7 days. Among clinical items of each score, duration of symptoms, previous TIA, hemiparesis, speech disturbance, gait disturbance, previous cerebral ischemic lesions, and known carotid artery disease were independent predictors of stroke. Clinical scores have moderate prognostic accuracy for stroke after TIA. Considering the independent predictors for stroke, our study indicates the need to continue research and prompts the development of new tools on predictive scores for TIA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transient ischemic attack (TIA) is a temporary episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction or tissue injury [1]. Ischemia results from a critical reduction of cerebral blood flow due to local (e.g., atherosclerosis, inflammation, amyloid deposition, and arterial dissection) or systemic (e.g., cardiac embolism) mechanisms. Symptoms usually last less than an hour, suggesting a short-lived dysfunction of an area of the central nervous system. TIA is a common neurologic disorder [2] with a reported overall prevalence of 2% and an estimated incidence of 240,000 TIAs per year and an average annual risk for a subsequent ischemic stroke of 3–4% in the United States [3, 4], with incidence varying with age, reported equal to 0.52–2.37 and 0.05–1.14 in men and women aged 55–64, 0.94–3.39 and 0.71–1.47 in those aged 65–74, and 3.04–7.20 and 2.18–6.06 in those aged 75–84, respectively in the European population [5]. The diagnosis is established by clinical features and neuroimaging findings [6]. Since 2009, the American Heart Association (AHA) has replaced the classic “time-based” definition of TIA, centred on the short duration of symptoms, with the “tissue-based” definition, highlighting that even the short duration of symptoms can be due to permanent brain damage and that the use of neuroradiology tests is a fundamental step in the diagnostic process [1]. Conversely, The European Stroke Organization (ESO) defines TIA “as transient neurological symptoms, likely to be due to focal cerebral or ocular ischemia, which last less than 24 h” [7], resulting in high heterogeneity in the literature among published studies on TIA. However, a key aspect of the diagnosis of TIA is attributing symptoms to cerebral ischemia despite the absence of neuroradiological findings. Although clinical features may be non-specific, ischemic insult is the most likely cause when the attack is consistent with TIA being characterized by focal neurologic symptoms attributable to a single vascular territory. Transient ischemic attack can be considered a serious warning for an imminent ischemic stroke, with the highest risk in the first 48 h. Physicians should identify high-risk TIA patients and establish how quickly they should receive specialist assessment, brain-neurovascular imaging, and cardiac evaluation. Methods that can reliably assess the risk of stroke after TIA would be useful for triaging patients and guide the timing and setting of diagnostic/therapeutic strategies. The age, blood pressure, clinical features, duration of symptoms, and diabetes (ABCD2) is one of the most used assessment scores being an easy tool applied to identify patients at high risk of ischemic stroke in the first 7 days after TIA [8]. However, over the years, the ABCD2 predictive performance has been questioned as this score failed to reliably distinguish low- from high-risk subsets of patients with TIA [9]. Moreover, the predictive power of the ABCD2 score is generally lower in in-hospital patients compared to population-based settings, thus hampering the validity of this test in high-risk populations [10]. Furthermore, ABCD2 is based on the “time-based” definition of TIA [9]. Indeed, there is now evidence that findings indicative of acute ischemic lesions at diffusion-weighted magnetic resonance imaging (DWI-MRI) or acute or chronic ischemic lesions at computed tomography (CT) scan after a transient ischemic event are important predictors of stroke [11,12,13]. Risk models that combine information from acute DWI-MRI, non-invasive angiography, and presumed TIA aetiology could improve the accuracy of stroke risk prediction after TIA. In addition to ABCD2, many other risk stratification scores have been developed for TIA/stroke, i.e., the ABCD2-I that includes the ABCD2 items along with information about brain infarction detected at DWI-MRI or CT [13], and the recently published OTTAWA score that considers brain imaging, clinical features, and laboratory findings [14]. The ABCD3-I has been demonstrated to be superior to the ABCD2 and ABCD2-I scores [15, 16]. However, the ABCD3-I requires the inclusion of the results of the DWI-MRI, an imaging test not commonly available for evaluating patients with TIA in most EDs. Current European [7] and American [17] guidelines on TIA and stroke management, however, do not support the use of any clinical risk prognostic scores in the initial triage due to the lack of robust evidence on their use and the scarcity of recent studies comparing them. However, clinical prediction rules are extremely useful in the management of patients based on individual risk and are widely used in clinical practice. This study aimed to compare the prognostic accuracy of the ABCD2, ABCD2-I, and OTTAWA scores in the prediction of stroke within 7 and 90 days as well as 1 year in patients presenting with TIA in the Emergency Department (ED). Secondary outcomes are the evaluation of clinical characteristics, duration of symptoms, and the therapy used as prognostic factors.
Methods
This is a retrospective, single-centre, 2-year cohort study. Our institutional electronic database was interrogated to enlist all patients aged > 18 years admitted to the ED of Arcispedale St. Anna, a referral centre for stroke in the Ferrara district, Cona, Ferrara, Italy, from January 1st, 2018, to December 31st, 2019, for “acute neurologic defect” or “TIA”. Only patients with a final diagnosis of TIA were included. According to our hospital protocol, all TIA diagnoses were established by neurological consultancy, using the “tissue-based” definition. All patients classified as TIA had no neurologic symptoms at the ED presentation, reported symptoms lasting < 24 h, and underwent a brain CT scan to exclude acute ischemic or haemorrhagic lesions. Patients with no neurologic symptoms and a new ischemic lesion on neuroimaging compatible with symptoms reported were defined as a “minor stroke” and excluded from the study. Clinical data were retrospectively extracted from our institutional electronic database including demographics, presenting, and accompanying symptoms, medical history, vital signs (blood pressure, heart rate, and peripheral blood oxygen saturation [SpO2]), and those specified by the ABCD2, ABCD2-I, and OTTAWA scores. The ABCD2 score, ABCD2-I, and OTTAWA scores were calculated in each patient according to the original studies [7, 12, 13] (see Table 1) on ED admission. The occurrence of stroke was defined by the re-admission to the ED with a new neurological defect and the presence of an ischemic lesion at neuroimaging, with the final diagnosis confirmed by a neurologist. Time points (i.e., 7 days, 90 days, and 1 year) were calculated from the date of the TIA leading to the first ED presentation.
Normally distributed data were described as mean and standard deviation (SD); not normally distributed data were described as the median and interquartile range (IQR); categorical data were reported as absolute numbers and percentages. Normally distributed data were compared via independent sample t test or Welch’s t test in case of unequal variance between groups. Not normally distributed data were compared via Mann–Whitney Test U. Pearson’s Chi-square test was used to compare categorical dependent variables among at least 2 independent groups.
The predictive ability of the scores was tested using the evaluation of the area under the receiver-operating characteristic (AUROC) curve. The AUROCs of the scores were compared using the method proposed by DeLong et al. [18]. The criterion associated with the highest Youden Index was considered the best cut-off. Multiple logistic regression analysis of each score (ABCD2; ABCD2;-I, and OTTAWA score) was performed for each outcome to evaluate the independent predictive power of each item.
Statistical analyses were performed using SPSS v.23 (Apache Software Foundation, Chicago, Illinois, USA) and MedCalc Version 17.6 (MedCalc Software BVBA). This study was approved by the Local Ethics Committee and conducted following the Helsinki Declaration.
Results
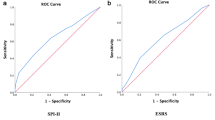
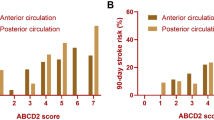
A total of 650 patients were initially included; of these, 147 patients were excluded, being classified as “acute neurologic deficit” but non-confirmed as a TIA by the neurologist. Finally, 503 patients were included in the study, 259 (51.5% male), with a median age of 77 years (IQR 25–75% 63–83 years). No patient had a haemorrhagic stroke or died during the year of observation. We found that 1.7% of patients developed a stroke within 7 days, 4.7% within 90 days, and 7.7% at 1 year from admission to the ED for TIA. As shown in Table 2, patients with hypercholesterolemia, previous TIA or stroke, carotid artery disease, duration of symptoms > 10 min and > 60 min, hemiparesis, and gait impairment were at higher risk of stroke. Conversely, patients with a previous or new chronic therapy with a statin, a beta-blocker, or low-weight molecular heparin and patients with a duration of symptoms < 10 min developed a significantly lower number of subsequent strokes. No differences were noted in the percentage of admitted patients between TIA patients who no developed stroke and patients who developed a stroke at each time-point. Clinical prediction scores were significantly higher in patients who developed stroke, while no differences were noted in diagnostic accuracy for the different outcomes, with AUROCs between 0.66 and 0.75. Evaluating the cut-offs of each score for each clinical outcome, only ABCD2 (with a score > 3) showed a sensitivity of 100% for stroke within 7 days, with an NPV of 100% (95% CI 97–100%) (Table 3 and Fig. 1). The multivariate analysis of each score showed the following results: for the ABCD2, “Hemiparesis” was the only independent predictor of stroke at each time-point, whereas both “Duration 10–59 min” and “Duration > 60 min” were independently predictive of stroke occurrence within 1 year. Among the item of the ABCD2-I, “Hemiparesis” was an independent predictor of stroke at each time-point, “Duration 10–59 min” and “Duration > 60 min” were independently predictive of stroke occurrence within 1 year, while “Ischemic lesion on head CT or MRI” was an independent predictor only with respect to stroke occurrence within 90 days. Finally, for the OTTAWA score, “Hemiparesis” was the only predictor of stroke within 7 days, whereas “Hemiparesis” and “Gait disturbance” were predictors of stroke within 90 days, and “Hemiparesis”, “Gait disturbance”, “First episode of TIA”, “ > 10-min symptom duration”, and “Known carotid artery disease” were independent predictors of stroke at 1 year (Table 4).
AUROCs of investigated scores at each time-point (7, 90 days and 1 year). For stroke within 7 days, ABCD2, ABCD2-I, and OTTAWA scores had an AUROC 0.73 (95% CI 0.61–083), 0.75 (95% CI 0.61–0.88), and 0.66 (95% CI 0.48–0.83), respectively; for stroke within 90 days, an AUROC of 0.75 (95% CI 0.71–0.78), 0.74 (95% CI 0.71–0.87), and 0.71 (95% CI 0.67–0.75), respectively; for stroke within 1 year, an AUROC of 0.69 (95% CI 0.65–0.73), 0.69 (95% CI 0.64–0.72), and 0.70 (95% CI 0.66–0.74), respectively
Discussion
Clinical scores provide a probability estimate of adverse events by assigning a specific score to some clinical and laboratory parameters [19]. Clinical scores were reported to be superior to isolated clinical judgment, because they collect the experience of many clinical cases and can objectively weigh the role of each item in the construction of the overall risk of a short-term adverse event [20, 21]. However, as demonstrated by Liao and Mark [20], physicians seem reluctant to use scores. One possible explanation is that there are many clinical prediction scores and identifying the best one in terms of ease of use and prognostic accuracy is often difficult. According to Chaudhary et al. [22], clinical scores developed for the prediction of stroke after a TIA are highly heterogeneous in terms of methodologies (i.e., different diagnostic criteria, e.g., “time-based” vs. “tissue-based”) and wide variability of the investigated patients (TIA or stroke or a combination of TIA and stroke patients). Also, Perry et al. [23] reported that the median sensitivity of clinical scores for TIA expected by physicians was higher than that reached by any existing scores, thus limiting their value in daily practice. An early diagnosis of TIA and a correct evaluation of several cardiovascular risk factors may aid adequate patient management leading to reduced rates of stroke, myocardial infarction, and vascular death as well as improved quality of life [24, 25]. According to our results, ABCD2, ABCD2-I, and OTTAWA risk scores have moderate diagnostic accuracy, with an AUROC < 0.75 in predicting the occurrence of stroke within 7 and 90 days, and at 1 year. In addition to the complexity of the OTTAWA score (which includes clinical, anamnestic, and laboratory data), there is no significant difference between this score and the ABCD2 and the ABCD2-I for all outcomes. As highlighted by the PROMAPA study [26], clinical scores were not able to replace a diagnostic evaluation, including blood tests, neuroradiologic and vascular imaging, and cardiac monitoring. Weimar et al. and Zhao et al. reported a low accuracy of clinical predictive scores for stroke [27, 28]. Specifically, Weimar et al. [27], who conducted a prospective cohort study in 16 German neurology departments, recruited 1897 consecutive patients with TIA or acute stroke and showed that all clinical predictive scores had an AUROC < 0.65 with low sensitivity and specificity. To assess the power of stroke prediction of ABCD2, Zhao et al. [28] performed a diagnostic meta-analysis and applied the results to a hypothetical cohort of 1000 patients with TIA. The pooled data of ABCD2 at 7 and 90 days showed a sensitivity of 79.9% and 76.6%, respectively, and a specificity of 29.2% and 40.3%, respectively. A recent paper by Perry et al. [29], including 7607 patients from 13 Canadian EDs, identified an AUROC for the OTTAWA TIA risk score of 0.70 (95% CI 0.66–0.73), which is a finding comparable to ours. However, while Perry et al. demonstrated that the OTTAWA score was significantly higher than ABCD2 (AUROC of 0.60; 95% CI 0.55–0.64) in predicting stroke at 1 week, our data revealed no significant difference among the investigated scores. Considering the best cut-off of each score, ABCD2 > 3 showed a sensitivity of 100% in the prediction of stroke within 7 days from the occurrence of TIA, with a negative predictive value (NPV) of 100%. This suggests that ABCD2 can be useful in excluding patients having a stroke in the short term (7 days). However, the limited number of patients enrolled in this study and the relatively low number of ischemic events prevent us to drawn firm conclusions on the NPV of ABCD2 in the short term. Since ABCD2-I and OTTAWA showed an NPV < 99% in the short, medium, and long term, these scores should not be used in excluding patients at risk of stroke occurrence.
Concerning the items of ABCD2 and ABCD2-I scores, only hemiparesis was an independent predictor of stroke, whereas hemiparesis and speech disturbance were independent predictors of stroke within 90 days. Regarding OTTAWA, hemiparesis predicted all outcomes, whereas the duration of symptoms and the known carotid artery disease were predictors of stroke within 1 year. Various authors have suggested adding the evaluation of brain or carotid imaging to the clinical scores to improve their diagnostic accuracy [30, 31]. However, in our series, the presence of any ischemic lesions at CT scan was an independent predictor of stroke in the subsequent 90 days and the presence of known carotid artery stenosis was a significant predictor within 90 days and at 1 year without increasing the accuracy of the ABCD2-I and OTTAWA vs. ABCD2. The multivariate analysis of each score at each time-point showed that despite the complexity of these scores, only a few elements appeared useful in identifying patients at higher risk of stroke. Moreover, as indicated in Table 2, cardiovascular risk factors and therapies have a potential role in the development of stroke at 90 days and 1 year. Thus, an ideal score that considers the right clinical elements, risk factors, and long-term therapies are expected to better predict the probability of a subsequent stroke.
Although this study is one of the few comparing the most applied clinical predictive scores for TIA in EDs, it has some limitations due to the retrospective nature of our database. First, the restricted access to the full set of patients’ information, including other imaging tests or exams performed, may have underestimated the total number of strokes in the 2-year investigational period. Second, despite the TIA protocol in our hospital being based on major guidelines for stroke, the decision to start a treatment or skip diagnostic investigations may have been taken on a case-by-case scenario, thus affecting the outcome. Third, the ABCD2-I original study [13] relies on the “time-based” TIA definition and assigned three points for a new ischemic lesion at brain DWI-MRI or any ischemic lesion at brain CT; however, patients with a new ischemic lesion compatible with the reported symptoms were considered as “minor stroke” and excluded from the study as potential confounders affecting the diagnostic accuracy of the score. Since no patients underwent DWI-MRI, a new ischemic lesion could remain undetected, thereby potentially affecting the “tissue-based” TIA definition used in our study. However, DWI-MRI is rarely performed in the EDs for TIA and all included patients also fulfilled the “time-based” definition of TIA. Finally, the limited number of patients with ischemic stroke in relation to the study endpoints (i.e., 7 days, 90 days, and 1 year) is likely to have downsized the statistical power of the study.
Conclusions
In conclusion, clinical prediction scores may be useful in managing patients with TIA in the ED and help stratify patients according to individual risk of stroke; however, this work showed that clinical scores have only moderate prognostic accuracy for stroke after TIA, with no differences among them at any time-point. Considering the independent predictors for stroke, our study indicates the need to continue research and prompts the development of new tools on predictive scores for TIA.
References
Easton JD, Saver JL, Albers GW et al (2009) Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American heart association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council On Cardio. Stroke 40(6):2276–2293. https://doi.org/10.1161/STROKEAHA.108.192218
Virani SS, Alonso A, Benjamin EJ et al (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141(9):e139. https://doi.org/10.1161/CIR.0000000000000757
Johnston SC, Fayad PB, Gorelick PB et al (2003) Prevalence and knowledge of transient ischemic attack among US adults. Neurology 60(9):1429. https://doi.org/10.1212/01.wnl.0000063309.41867.0f
Kleindorfer D, Panagos P, Pancioli A et al (2005) Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 36(4):720. https://doi.org/10.1161/01.STR.0000158917.59233.b7
Kokubo Y (2014) Epidemiology of transient ischemic attack. Front Neurol Neurosci 33:69–81. https://doi.org/10.1159/000351892
Sorensen AG, Ay H (2011) Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am 21(2):303. https://doi.org/10.1016/j.nic.2011.01.013
Fonseca AC, Merwick Á, Dennis M et al (2021) European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur Stroke J. https://doi.org/10.1177/23969873211027003
Johnston SC, Rothwell PM, Nguyen-Huynh MN et al (2007) Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 369(9558):283. https://doi.org/10.1016/S0140-6736(07)60150-0
Wardlaw JM, Brazzelli M, Chappell FM et al (2015) ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1000 patients triaged. Neurology 85(4):373. https://doi.org/10.1212/WNL.0000000000001780
Stead LG, Suravaram S, Bellolio MF et al (2011) An assessment of the incremental value of the ABCD2 score in the emergency department evaluation of transient ischemic attack. Ann Emerg Med 57(1):46. https://doi.org/10.1016/j.annemergmed.2010.07.001
Al-Khaled M, Eggers J (2013) MRI findings and stroke risk in TIA patients with different symptom durations. Neurology 80(21):1920–1926. https://doi.org/10.1212/WNL.0b013e318293e15f
Sciolla R, Melis F, SINPAC Group (2008) Rapid identification of high-risk transient ischemic attacks: prospective validation of the ABCD score. Stroke 39(2):297. https://doi.org/10.1161/strokeaha.107.496612
Giles MF, Albers GW, Amarenco P et al (2010) Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke 41(9):1907. https://doi.org/10.1161/STROKEAHA.110.578971
Perry JJ, Sharma M, Sivilotti ML et al (2014) A prospective cohort study of patients with transient ischemic attack to identify high-risk clinical characteristics. Stroke 45(1):92–100. https://doi.org/10.1161/strokeaha.113.003085
Knoflach M, Lang W, Seyfang L et al (2016) Predictive value of ABCD2 and ABCD3-I scores in TIA and minor stroke in the stroke unit setting. Neurology 87(9):861–869. https://doi.org/10.1212/WNL.0000000000003033
Xie X, Jing J, Meng X et al (2021) Predictive value of the ABCD3-I for short- and long-term stroke after TIA with or without sICAS. J Atheroscler Thromb. https://doi.org/10.5551/jat.63050.10.5551/jat.63050 (published online ahead of print, 2021 Nov 6)
Powers WJ, Rabinstein AA, Ackerson T et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50(12):e344–e418. https://doi.org/10.1161/STR.0000000000000211
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845 (PMID: 3203132)
Randolph A, Guyatt H, Calvin JE, Doig G, Richardson WS (1998) Understanding articles describing clinical prediction tools. Crit Care Med 26:1603–1612. https://doi.org/10.1097/00003246-199809000-00036
Liao L, Mark DB (2003) Clinical prediction models: are we building better mousetraps? J Am Coll Cardiol 42:851–853. https://doi.org/10.1016/s0735-1097(03)00836-2
Gandara E, Wells PS (2010) Diagnosis: use of clinical probability algorithms. Clin Chest Med 31:629–639. https://doi.org/10.1016/j.ccm.2010.07.002
Chaudhary D, Abedi V, Li J, Schirmer CM, Griessenauer CJ, Zand R (2019) Clinical risk score for predicting recurrence following a cerebral ischemic event. Front Neurol 10:1106. https://doi.org/10.3389/fneur.2019.01106
Perry JJ, Mansour M, Sharma M et al (2010) National survey of Canadian neurologists’ current practice for transient ischemic attack and the need for a clinical decision rule. Stroke 41(5):987–991. https://doi.org/10.1161/strokeaha.109.577007
Cocho D, Monell J, Planells G et al (2016) Rapid diagnosis and treatment of TIA results in low rates of stroke, myocardial infarction and vascular death. Neurologia 31(1):18–23. https://doi.org/10.1016/j.nrl.2014.05.005
Sangha RS, Caprio FZ, Askew R et al (2015) Quality of life in patients with TIA and minor ischemic stroke. Neurology 85(22):1957–1963. https://doi.org/10.1212/WNL.0000000000002164
Purroy F, Jiménez Caballero PE, Gorospe A et al (2012) Prediction of early stroke recurrence in transient ischemic attack patients from the PROMAPA study: a comparison of prognostic risk scores. Cerebrovasc Dis 33(2):182–189. https://doi.org/10.1159/000334771
Weimar C, Benemann J, Michalski D et al (2010) Prediction of recurrent stroke and vascular death in patients with transient ischemic attack or nondisabling stroke: a prospective comparison of validated prognostic scores. Stroke 41(3):487–493. https://doi.org/10.1161/strokeaha.109.562157
Zhao M, Wang S, Zhang D et al (2017) Comparison of stroke prediction accuracy of ABCD2 and ABCD3-I in patients with transient ischemic attack: a meta-analysis. J Stroke Cerebrovasc Dis 26(10):2387–2395. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.05.030
Perry JJ, Sivilotti MLA, Émond M et al (2021) Prospective validation of Canadian TIA Score and comparison with ABCD2 and ABCD2I for subsequent stroke risk after transient ischaemic attack: multicentre prospective cohort study. BMJ 372:n49. https://doi.org/10.1136/bmj.n49
Kelly PJ, Albers GW, Chatzikonstantinou A et al (2016) Validation and comparison of imaging-based scores for prediction of early stroke risk after transient ischaemic attack: a pooled analysis of individual-patient data from cohort studies. Lancet Neurol 15(12):1238–1247. https://doi.org/10.1016/S1474-4422(16)30236-8
Kiyohara T, Kamouchi M, Kumai Y et al (2014) ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke 45(2):418–425. https://doi.org/10.1161/strokeaha.113.003077
Acknowledgements
We would like to thank Elena Forini of the Health Statistics Office, Planning and Management Control Department of St. Anna Hospital, Cona, Ferrara, for providing the data assessed in this study. Also, we wish to thank Dr. Roberto Zoppellari, MD, director of the Emergency Department of the St. Anna Hospital, Cona, Ferrara, for insightful comments and clinical support to the present study.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. No funding was received to conceive, draft, or publish this manuscript.
Author information
Authors and Affiliations
Contributions
MDS, AR, AS, and RDG conceived the study. MDS, BM, CG, and ISF organised and analysed the data. MDS, MG, and AP drafted the manuscript. MC, AG, and FF revised the data and the manuscript. MDS and RDG drafted the final version of the manuscript. All authors reviewed and accepted the final version of the manuscript. The authors declare that they have no conflict of interest to declare. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Human and animal rights statement
The paper was made in accordance with the declaration of Helsinki and approved by the local Internal Review Boards.
Informed consent
Given its retrospective and anonymized database the informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spampinato, M.D., Covino, M., Passaro, A. et al. ABCD2, ABCD2-I, and OTTAWA scores for stroke risk assessment: a direct retrospective comparison. Intern Emerg Med 17, 2391–2401 (2022). https://doi.org/10.1007/s11739-022-03074-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-03074-x