Abstract
Central venous pressure (CVP) is primarily measured to assess intravascular volume status and heart preload. In clinical practice, the measuring device most commonly used in emergency departments and intensive care units, is an electronic transducer that interconnects a central venous catheter (CVC) with a monitoring system. Non-invasive ventilation (NIV) consists in a breathing support that supplies a positive pressure in airways through a mask or a cask though not using an endotracheal prosthesis. In emergency settings, non-invasive ultrasonography evaluation of CVP, and hence of intravascular volume status entail the measurement by a subxiphoid approach of inferior vena cava diameter and its variations in relation to respiratory activity. In the literature, there are many studies analyzing the ability to estimate CVP through ultrasonography, rating inspiratory and expiratory vena cava diameters and their ratio, defined as inferior vena cava collapsibility index (IVC-CI). At the same time, the effects of invasive mechanical ventilation on blood volume and the correlation during ventilation between hemodynamic invasive measurement of CVP and inferior vena cava diameters have already been demonstrated. Nevertheless, there are no available data regarding the hemodynamic effects of NIV and the potential correlations during this kind of ventilation between invasive and non-invasive CVP measurements. Therefore, this study aims to understand whether there exists or not an interrelationship between the values of CVP assessed invasively through a CVC and non-invasively through the IVC-CI in patients with severe respiratory distress, and above all to evaluate if these means of assessment can be influenced using NIV.
Similar content being viewed by others
Introduction
Central venous pressure (CVP), also known as right atrial pressure, represents blood pressure in the thoracic vena cava close to the right atrium and reflects the amount of blood returning to the heart; moreover, the ability of the left ventricle to pump into the systemic arterial system, whereby CVP is determined by the interaction of cardiac function and return function. A change in either can alter it. Furthermore, a certain number of extrinsic factors can alter the precision in evaluating CVP [1–3]. Among those factors there are the mechanisms that increase thoracic pressure, for example, cough, effort and positive pressure ventilation [4]. Some fluctuations of the CVP wave can also be found in patients with a significant tricuspid regurgitation or atrioventricular dissociation [5]. Historically, the devices monitoring CVP invasively have been considered the standard in intravascular volume evaluation, but there is no consensus for their indications as well as for the exactness of the so obtained values [6, 7]. Furthermore, the devices used for invasive monitoring can lead to complications due both to their insertion procedure and their use. For these reasons during the past years, non-invasive methods have been investigated for their potential in assessing the patient’s volume status and cardiac function [8]. During emergency ultrasonography, the non-invasive volume status evaluation consists in the inferior vena cava (IVC) diameter assessment and its variations during respiratory activity through a subxiphoid view. This approach provides a reliable estimate of right atrium pressure, and therefore of central venous pressure. Even if the ultrasonographic evaluation particularly in an acute respiratory distress patients, is difficult due to non-collaboration by the patient or abdominal wall involvement during respiratory effort, as required by the ACEP guidelines, [9] a good training can help overtaking these difficulties. The first studies regarding caval diameters and CVP estimate as assessing methods for intravascular volume status have been conducted in the 1990s, highlighting how IVC diameters might reflect hemodynamic, right heart function and blood volume. During inspiratory time, a negative pressure develops in the pleural space, leading to an increase in right heart preload. Therefore, augmenting blood flow in the inferior vena cava and diminishing intraluminal pressure, the vessel diameter decreases [10]. Later, other parameters have been evaluated to better estimate CVP, for example, inferior vena cava collapsibility index (IVC-CI), which represents the difference between end-expiratory and end-inspiratory IVC diameter, divided by tele-expiratory diameter. Some studies have proved how IVC-CI correlates with CVP, in particular, reporting that elevated values of IVC-CI reflect low values of CVP and vice versa [11, 12].
Even if numerous studies have been conducted to correlate caval diameters with CVP in patients spontaneously breathing [13], there are not many works about patients invasively ventilated. With regard to non-invasive ventilation (NIV), nowadays there are no data in the literature about its hemodynamic repercussions and its potential effects in the correlation between invasive and ultrasonography assessment of CVP. The aim of this study is to evaluate whether there exists or not a correlation between the values of CVP estimate invasively through a central venous catheter (CVC), and non-invasively (through IVC-CI) in patients presenting to the emergency department (ED) with respiratory distress, and whether positive pressure ventilation can influence those assessing methods.
Methods
A prospective accuracy study was performed at the ED of an urban academic level I trauma centre. The study, which is consistent with the principles of the Declaration of Helsinki on clinical research involving human subjects, was approved by an Institutional Review Board.
Adult patients consecutively presenting at the ED of Careggi University Hospital with severe respiratory distress due to various causes, and requiring the use of mechanical non-invasive positive pressure ventilation, have been enrolled in this study.
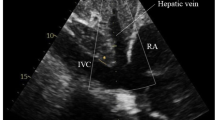
Exclusion criteria were pregnancy and shock, defined by the presence of an arterial systolic blood pressure inferior to 90 mmHg. For each patient, a written informed consent to the enrolment in this study and the use of personal data has been obtained. The study has been carried out by a group of ten physicians working in the emergency department (including also emergency residents attending at least the third year of residency). They have been previously trained in the use of ultrasonography in emergency both with 80 h of theoretical lessons and a practical training during which everyone has accomplished at least 150 thoracic ultrasonography and 150 cardiac ultrasonography (as required by the ACEP guidelines) [9]. Patients enrolled in the study underwent a routine work-up for respiratory distress, including monitoring of the electrocardiogram (EKG) as well as pulse oximetry and arterial blood gas analysis (BGA). For patients requiring an evaluation of CVP due to their respiratory or hemodynamic conditions, a central triluminal venous catheter (Blue FlexTip catheters, Arrow-Howes, Reading, PA, USA, with a diameter of 7 French and a length of 20 cm) was placed in the internal jugular vein under ultrasonography guide, or in the subclavian vein using anatomical markers as reference points. An echocardiography was always performed before the beginning of ventilation. From the apical four chamber view, the physicians were able to assess the ejection fraction (EF) by means of the Simpson method; through a subxiphoid approach, they evaluated the IVC in a two-dimensional modality (B-Mode). The IVC was then assessed proximally to the junction with the hepatic vein that arises about 2 centimeters distal to the right atrium through a mono-dimensional imagine (M-Mode). Monitoring the EKG simultaneously to the ultrasound examination made possible the recording of the correct caval diameters at the end of diastole during expiratory time and at the end of systole during inspiration. In fact, the use of the R-wave as an index of end diastole, allows avoidance of the tricuspid valve regurgitation, and consequently the caval expiratory reflux often observed during mechanical ventilation [14], which according to the literature, is considered able to affect the inferior vena cava diameters [15]. The positive pressure ventilation thereafter began with a Dräger Carina ventilator (Drägerwerk AG & Co. KGaA Moislinger Allee 53–55 Lübeck, 23558), was performed in the most appropriate modality depending on the effective patient’s requirements: continuous positive airway pressure (CPAP) with a positive end-expiratory pressure (PEEP), or CPAP-PS adding to the PEEP a pressure support (PS) [16, 17]. To evaluate the influence of positive pressures on CVP, we decided to use the only parameter present both in CPAP and CPAP-PS modality, and therefore the different PEEP values. During the first 30 min of ventilation, the maximum volume charge accepted was 500 ml, and there were no introduction or modifications in the dosage of drugs such as vasopressors/vasodilators. A new ultrasound evaluation of heart and inferior vena cava was performed with the same modalities at 30 min from the beginning of ventilation. The ultrasonography was a bedside examination, executed with a Philips iE33 ultrasound machine (Philips Ultrasound, Bothell, WA, USA) and a MyLab 30 Gold ultrasonography unit (Esaote, Genoa, Italy), both equipped with a sector ultrasound transducer of 2.5–3.5 MHz. Before starting ventilation and 30 min after, were collected also oxygen saturation (SpO2), percentage of oxygen in inhaled gas (FiO2), respiratory rate (RR), heart rate (HR), arterial systolic (SBP) and diastolic (DBP) blood pressure, pH, pO2, pCO2, HCO3 −, lactates, CVP, inferior vena cava diameters and ejection fraction (EF).
Statistic analysis
The continuous parameters are reported as mean ± standard deviation while nonparametric values are reported as absolute value and percentage. Parameter variations detected during non-invasive and spontaneous breathing after 30′ ventilation were analyzed using the Student’s t test for paired data. The comparison of percentages was performed with Pearson’s Chi-squared test. The correlation between continuous parameters was analyzed by linear regression and the standard error of the equation (SEE) calculation. To verify the presence of a systematic error in the estimate or percentage of CVP values from the diameter of the IVC was used Bland–Altman analysis and the calculation of the confidence limits (95%) average error. The p value was calculated using the two-tailed test and a p value of <0.05 was considered statistically significant. The Bland–Altman analysis was performed with GraphPad Prism statistical program. Statistical analysis was performed using SPSS package program, version 19 (SPSS Inc., Chicago, IL, USA).
Results
Between January 2013 and December 2014, 160 patients were enrolled in our study. 34 patients were excluded because the placement of a CVC was not necessary; two were taken out because they were not able to tolerate positive pressure ventilation for at least 30 min. Of the 124 remaining patients, 66 were male and 58 female, with a medium age of 79 ± 8 years (range 59–93 years). The central venous line was placed in the internal jugular vein for 86 patients and in the subclavian vein through an infraclavicular approach for 38 patients. At the initial evaluation, 40 (32%) patients had a partial respiratory insufficiency (hypoxemic), while 84 (68%) had a global respiratory failure (hypoxemic and hypercapnic). As reported in Table 1 at the beginning of ventilation, the patients had a RR of 31 ± 7 brpm, an HR of 106 ± 17 bpm, a SBP of 135 ± 32 mmHg, a DBP of 75 ± 19 mmHg, a CVP of 8 ± 4 mmHg, and an EF of 46 ± 15%. Twenty-six patients were ventilated using only an expiratory positive airway pressure (EPAP) (therefore with a PEEP) of 8 ± 2 mmHg, while in 98 patients the ventilation system was set with an inspiratory positive airway pressure (IPAP) (therefore PEEP + PS) of 21 ± 5 mmHg. In all patients, ultrasonographic assessment was possible. The linear regression analysis on parameters assessed during spontaneous breathing shows a significant correlation between CVP and the diameter of IVC in expiratory and inspiratory period. In Fig. 1 illustrates the high correlation between CVP and IVC-CI with a SEE = 1.443 mmHg. After 30′ of non-invasive ventilation, the same linear regression analysis shows a significant correlation between CVP, expiratory and inspiratory IVC diameters; specifically a high correlation is seen between CVP and IVC-CI (Fig. 2) with a SEE = 1.518 mmHg. The application of NIV causes an elevation in CVP values of 1.74 ± 0.76 mmHg. Even though there is a significant correlation between the diameter of IVC assessed by ultrasonography and the CVP values measures invasively, the Bland–Altman analysis shows how the values of CVP calculated through the IVC diameters are significantly overestimated when invasive CVP values are normal or low, while when invasive CVP values are above the normal limit they tend to underestimate CVP (Fig. 3). We stratified patients into three groups by their IVC collapsibility index: high (IVC-CI ≥ 0.60), intermediate (IVC-CI 0.25–0.60) and low (IVC-CI < 0.25). Following this subdivision, with regard to baseline parameters, we observe that in the group with high IVC-CI 96% of patients have a CVP ≤5 mmHg, while 4% of patients have a CVP between 6 and 10 mmHg; in the intermediate group 14% of patients have a CVP ≤5 mmHg, 76% a CVP between 6 and 10 mmHg and 10% of patients have a CVP >10 mmHg; finally, in the group with low IVC-CI 6% of patients have a CVP of 6-10 mmHg while the remnant 94% have a CVP >10 mmHg. After 30 min of positive pressure ventilation, in the group with an high index, 86% of patients have a CVP ≤5 mmHg while 14% have a CVP between 6 and 10 mmHg; in the group with intermediate collapsibility index 11% of patients have a CVP ≤5 mmHg, 78% a CVP between 6 and 10 mmHg, and 11% of patients a CVP >10 mmHg; in the group with low IVC-CI, 13% of patients have a CVP between 6 and 10 mmHg while the other 87% of patients have a CVP >10 mmHg (Fig. 4). Finally, analyzing the influence of positive end-expiratory pressures on CVP values variations, we note no significant correlation between CVP values and different PEEP supplied to the patients (Fig. 5).
Discussion
Our prospective study has attempted to evaluate whether or not there exists a correlation between central venous pressure values estimated invasively (CVC) and non-invasively (IVC-CI) in patients presenting to the ED with respiratory distress, and whether NIV can influence those assessing methods. It has already been demonstrated how IVC-CI inversely correlates with CVP [11, 12]. In trying to demonstrate a correlation between CVP and IVC-CI, Stawicki et al. in a study of 2009 [18] subdivided patient in three groups with high (>0.60), intermediate (0.20–0.60) or low (<0.20) collapsibility index, observing values of CVP, respectively, of 7.40 ± 4.67, 9.75 ± 5.23 and 12.00 ± 5.56. Despite a review conducted in 2008 [19], to clarify the usefulness of CVP evaluating the response to fluid therapy, in which it is affirmed that analyzing the results obtained from different studies there is not an association strong enough between CVP and circulating blood volume, and therefore that CVP cannot predict fluid response in a wide spectrum of clinical conditions, the evaluation of CVP both invasively and non-invasively is still widely used in the management of fluid therapy in critically ill patients. Actually, there are not many studies regarding the relationship between CVP evaluation and invasive ventilation. The first three studies with regard to this subject were done at the beginning of the 1990s, and their conclusions were contradictory. In fact, while Lichtenstein et al. [20] conclude that during invasive ventilation, CVP correlates with inferior vena cava diameters; Jue et al. [21] as well as Nagueh et al. [22] observe an inadequate correlation between the same parameters. A possible explication for those different conclusions could be the use of different methods in evaluating IVC. With regard to this matter in 2002, Bendjelid et al. [23] observe that in ventilated patients, the relation between CVP and inferior vena cava diameters assessed through ultrasonography are influenced by the IVC evaluating technique used, concluding that the best methodology is utilizing a mono-dimensional image (M-Mode) combining ultrasonography and ECG monitoring, and is able to register the correct values of IVC diameter at end diastole and end systole, respectively, during expiratory and inspiratory period [24]. From then on, all the studies conducted made use of this technique, confirming how this combination of methods is able to correctly evaluate the IVC diameters [24, 25], and IVC-CI [26]. The results obtained from our data analysis demonstrate a good correlation between the traditional measurement of central venous pressure and the assessment of both expiratory and inspiratory IVC diameters during spontaneous breathing, shown as a high and significant correlation between CVP and IVC-CI (r = 0.879, p < 0.0001). In addition, after the beginning of positive pressure ventilation, the results obtained from our analysis seem to remain close to baseline ones: the correlation between CVP and IVC-CI is once again high (r = 0.865, p < 0.0001). When analyzed with Bland–Altman plot, those data contain a percentage error, showing an overestimation of normal-low values of CVP and an underestimation of high values of CVP. The evaluation of volume status in emergency settings is notoriously fundamental in patients management to stabilize rapidly, and with a good precision whether the central venous pressure is normal, low or high allows choosing the proper therapy for each critically ill patient. CVP is defined low (≤5 mmHg), normal (6–10 mmHg) or high (>10 mmHg), reflecting the patient’s volume status, respectively, hypovolemia, euvolemia and hypervolemia. With regard to the assessment of IVC-CI, we stratified our patients following an arbitrarily subdivision into three groups: high, intermediate and low collapsibility index. In patients breathing spontaneously, it is interesting to note how in the group with high IVC-CI there is a high percentage of patients (96%) with low CVP (≤5 mmHg), while vice versa in the low IVC-CI group there is a great number of patients (94%) with a high CVP (>10 mmHg). Furthermore, after 30 min of positive pressure ventilation in the same groups the respective percentages remain elevated. In regard to the different ventilation modalities used, as previously explained, we compared them considering the PEEP. From our study emerge no significant correlation between the use of different PEEP and CVP variations, emphasizing how IVC-CI is independent from the values of PEEP.
Limitations
We decided not to consider the different causes of respiratory distress because the goal of our study was to evaluate the relationship between positive pressure ventilation and CVP independently from the etiologies causing the respiratory failure. We mention only the different type of respiratory failure which affects our patients: partial respiratory or global respiratory failure.
Another limitation was that we did not evaluate the intra-observer variability in performing ultrasonography scan. Finally, in our study, we did not measure any parameter regarding the right heart.
Conclusion
Our study confirms the literature data regarding the correlation between expiratory and inspiratory IVC diameters and central venous pressure values, thus highlighting particularly a strong correlation between IVC-CI and CVP. Furthermore, we observe that there is no influence of NIV in non-invasive assessment of right atrium pressures in our patients. This is extremely important in the emergency medicine setting, where a rapid overview of patient volume status is fundamental for clinical and therapeutic management, where NIV is becoming widespread, and could therefore be used without altering patients’ hemodynamics.
References
Magder S (2005) How to use central venous pressure measurements. Curr Opin Crit Care 11(3)
Polanco PM, Pinsky MR (2006) Practical issues of hemodynamic monitoring at the bedside. Surg Clin North Am 86(6)
Magder S (2006) Central venous pressure: a useful but not so simple measurement. Crit Care Med 34(8)
Lansdorp B, Hofhuizen C, van Lavieren M, van Swieten H, Lemson J, van Putten MJ, van der Hoeven JG et al (2014) Mechanical ventilation-induced intrathoracic pressure distribution and heart-lung interactions. Crit Care Med 42(9):1983–1990
Magder S (2006) Central venous pressure monitoring. Curr Opin Crit Care 12(3)
Connors AF, Jr., Speroff T, Dawson NV, Thomas C, Harrell FE, Jr., Wagner D, et al (1996) The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 276(11)
Shah MR, Hasselblad V, Stevenson LW, Binanay C, O’Connor CM, Sopko G et al (2005) Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 294(13)
Shoemaker WC, Wo CC, Chien LC, Lu K, Ahmadpour N, Belzberg H et al (2006) Evaluation of invasive and noninvasive hemodynamic monitoring in trauma patients. J Trauma 61(4)
ACEP (2011) Emergency ultrasound fellowship guidelines. ACEP
Kircher BJ, Himelman RB, Schiller NB (1990) Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol 66(4)
Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S et al (2007) Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 20(7)
Ommen SR, Nishimura RA, Hurrell DG, Klarich KW (2000) Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc 75:24–29
De Lorenzo RA, Morris MJ, Williams JB, Haley TF, Straight TM, Holbrook-Emmons VL et al (2012) Does a simple bedside sonographic measurement of the inferior vena cava correlate to central venous pressure? J Emerg Med 42(4)
Jullien T, Valtier B, Hongnat JM, Dubourg O, Bourdarias JP, Jardin F (1995) Incidence of tricuspid regurgitation and vena caval backward flow in mechanically ventilated patients. A color Doppler and contrast echocardiographic study. Chest 107:488–493
Mohiaddin RH, Wann SL, Underwood R, Firmin DN, Rees S, Longmore DB (1990) Vena caval flow: assessment with cine MR velocity mapping. Radiology 177:537–541
British Thoracic Society Standards of Care Committee et al (2002) Non-invasive ventilation in acute respiratory failure. Thorax 57(3)
Mehta S, Hill NS (2001) Noninvasive ventilation. Am J Respir Crit Care Med 163(2)
Stawicki SP, Braslow BM, Panebianco NL, Kirkpatrick JN, Gracias VH, Hayden GE et al (2009) Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg 209(1)
Marik PE, Baram M, Vahid B (2008) Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 134(1)
Lichtenstein D, Jardin F (1994) Appréciation non-invasive de la pression veineuse centrale par la mesure échographique du calibre de la veine cave inférieure en réanimation. Réan Urg 3:79–82
Jue J, Chung W, Schiller NB (1992) Does inferior vena cava size predict right atrial pressures in patients receiving mechanical ventilation? J Am Soc Echocardiogr 5:613–619
Nagueh SF, Kopelen HA, Zoghbi WA (1996) Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation 93:1160–1169
Bendjelid K, Romand JA, Walder B, Suter PM, Fournier G (2002) Correlation between measured inferior vena cava diameter and right atrial pressure depends on the echocardiographic method used in patients who are mechanically ventilated. J Am Soc Echocardiogr 15(9)
Schefold JC, Storm C, Bercker S, Pschowski R, Oppert M, Kruger A et al (2010) Inferior vena cava diameter correlates with invasive hemodynamic measures in mechanically ventilated intensive care unit patients with sepsis. J Emerg Med 38(5)
Arthur ME, Landolfo C, Wade M, Castresana MR (2009) Inferior vena cava diameter (IVCD) measured with transesophageal echocardiography (TEE) can be used to derive the central venous pressure (CVP) in anesthetized mechanically ventilated patients. Echocardiography 26(2)
Barbier C, Loubières Y, Schmit C, Hayon J, Jardin F, Vieillard-Baron A (2004) Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 30:1740–1746
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zanobetti, M., Prota, A., Coppa, A. et al. Can non-invasive ventilation modify central venous pressure? Comparison between invasive measurement and ultrasonographic evaluation. Intern Emerg Med 12, 1279–1285 (2017). https://doi.org/10.1007/s11739-016-1574-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-016-1574-8