Abstract
Using 6 culture media (12, 12D, 12G, 11, A and B) made up of MS medium (Murashige-Skoog, 1962) supplemented or not with glycerine, with different cytokinins, and/or 2,4-D, the morphological characteristics and contents in total carbohydrates, reducing sugars, sucrose and starch were studied in calli induced from explants (cotyledon, petiole, hypocotyl and leaf) obtained from Medicago strasseri seedlings. Callus formation was induced under photoperiod (16h light/8h darkness) conditions or in the absence of light.
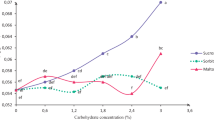
Considerable variability in the calli was observed, depending on the explants and media used. Under photoperiod conditions, medium A with KIN (1 mg/l) and 2,4-D (3 mg/l) induced many calli with the highest contents in total carbohydrates (886.1–889.3 mg/g DW), sucrose (132.1–188.2 mg/g DW) and starch (125.2–247.6 mg/g DW) and the lowest contents in reducing sugars (118.4–173.3 mg/g DW). In media 11, A and B, under conditions of darkness, calli degenerated at the start of culture. Calli developed in darkness generally had dry weights and total carbohydrate and starch contents lower than those cultured under photoperiod conditions. However, sucrose contents were greater in calli formed in darkness.
At these cultures times, differentiation, in the form of organogenesis, was only seen using medium B with cotyledons, petioles and leaves as explants. It was also observed when petioles were cultured in medium A but with a less pronounced organogenic response.
Similar content being viewed by others
Abbreviations
- C.H.:
-
carbohydrates
- D:
-
darkness
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- DW:
-
dry weight
- FW:
-
fresh weight
- KIN:
-
kinetin
- Ph:
-
photoperiodic conditions
- TDZ:
-
Thidiazuron
References
Branca C., Torelli A., Fermi P., Altamura M.M., Bassi M. 1994. Early phases “in vitro” culture tomato cotyledony: Starch accumulation and protin partten in relation to the horizontal treatment. Protoplasma 182, 59–64.
Dubois M., Gilles K.J., Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 356–361.
Gordon A.J., Ryle G.J.A., Mitchell D.F., Lowry K.H., Powell C.E. 1986. The effect of defoliation on carbohydrate, protein and leg-haemoglobin content of white clover nodules. Annals of Botany 58, 141–154.
Greuter W., Matthäs U., Risse H. 1982. Notes on Cardaegean plants. 3. Medicago strasseri, a new leguminous shrub from Kriti. Willdenowia 12,201–206.
Huber S.C., Akazawa T. 1986. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 81, 1008–1013.
Jenner C.F. 1982. Storage of Starch. In: Loevus FA and Tanner W (eds) Plant carbohydrates I, Intracellular carbohydrate. pp. 700–737. Springer-Verlag. Berlin.
Keller G.L., Nikolau B.J., Ulrich T.H. Wurtele E.S. 1988. Comparison of starch and ADP-glucose pyrophosphorylase levels in nonembryogenic cells and developing embryos from induced carrot cultures. Plant Physiol. 86, 451–456.
Komor E., Thom M., Maretzki A. 1981. The mechanism of sugar uptake by sugarcane suspension cells. Planta 153, 181–192.
Lai F-M., McKersie B.D. 1994. Regulation of starch and protein accumulation in alfalfa (Medicago sativa L.) somatic embryos. Plant Sci. 100, 211–219.
Mangat B.S., Pelekis M.K., Cassells A.C. 1990. Changes in the starch content during organogenesis in in vitro cultured Begonia rex stem explants. Physiol. Plant. 79, 267–274.
Murashige T., Skoog F. 1962. A revised medium for rapid growth and bioassays with tabacco tissue cultures. Physiol. Plant. 15, 473–497.
Naidu K.R., Kishor P.B.K. 1995. Activities of hydrolytic enzymes in callus cultures of tobacco during organogénesis. J. Bioscien. 20, 629–636.
Nelson N. 1944. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 153, 375–380.
Nguyen S.T., Paquin R. 1971. Méthodes d’extraction et de purification des acides aminés libres et des protéines de tissus végétaux. J. Chromatogr. 61, 349–351.
Paek K.Y., Canderlerd S., Thorpe T.A. 1988. Physiological effects of Na2SO4 and NaCl on callus cultures of Brassica campestris (Chinesse cabbage). Physiol. Plant. 72, 160–166
Preiss J. 1982. In: Loevus FA and Tanner W (eds) Plant carbohydrates I, Intracellular carbohydrate. pp. 397–417. Springer-Verlag. Berlin.
Rawal S.K., Dwivedi U.N., Khan B.M., Mascarenhas A.F. 1984. Biochemical aspects of shoot differentiation in sugarcane callus: Carbohydrate metabolizing enzymes. J. Plant Physiol. 119, 191–199.
Rout G.R., Samantaray S, Das P. 1995. Somatic embryogenesis and plant regeneration from callus culture of Acacia catechu — a multipurpose leguminous tree. Plant Cell, Tiss. Org. Cult. 42, 283–285.
Somogyi M. 1952. Notes on sugar determination. J. Biol. Chem. 195, 19–23.
Stamp J.A. 1987. Somatic embryogenesis in cassava. The anatomy and morphology of the regeneration process. Ann. Bot. 57, 451–459.
Swarnkar P.L., Bohra S.P., Chandra N. 1986. Biochemical studies on initiation of callus in Solanum surattense. J. Plant Physiol. 126, 293–296.
Thorpe T.A., Joy R.W., Leung D.W.M. 1986. Starch turnover in shoot-forming tobacco callus. Physiol. Plant. 66, 58–62.
Venkataramana S., Naidu K.M., Singh S. 1991. Invertases and growth factors dependent sucrose accumulation in sugarcane. Plant Sci. 74, 65–72.
Vu J.C.V., Niedz R.P., Yelonsky G. 1993. Glycerol stimulation of chlorophyll synthesis, embryogenesis, and carboxylation and sucrose metabolism enzymes in nucellar callus of “Hamlin” sweet orange. Plant Cell, Tiss. Org. Cult. 33, 75–80.
Vu J.C.V., Niedz R.P., Yelonsky G. 1995. Activities of sucrose metabolism enzymes in glycerol grown suspension cultures of sweet orange (Citrus sinensis L. Osbeck). Env. Exp. Bot. 35, 455–462.
Zheng, Q., Dessai, A.P., Prakash, C.S. 1996. Rapid and repetitive plant regeneration in sweetpotato via somatic embryogenesis. Pl. Cell. Rep. 15, 381–385.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Medina, M., Villalobos, N., De La Cruz, P.J. et al. Effect of culture medium and light conditions on the morphological characteristics and carbohydrate contents of Medicago strasseri calli. Acta Physiol Plant 20, 383–392 (1998). https://doi.org/10.1007/s11738-998-0024-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-998-0024-2