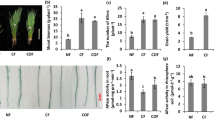
Abstract
Iron (Fe) deficiency chlorosis (FDC) in plant is associated with high bicarbonate concentration in calcareous soil and irrigation water, which leads to new leaf yellowing and lessens crop yield and quality. However, little is known about whether the chlorosis under bicarbonate stress resulted from blocking root–shoot Fe translocation or root Fe absorption. Moreover, the molecular response of chlorotic leaf under bicarbonate stress has been rarely reported on. The purpose of this study was to investigate the effect of bicarbonate on Fe acquisition, Fe translocation as well as Fe accumulation in roots, normal leaf (NL) and chlorotic leaf (CL) of Medicago lupulina. Seeds were grown with and without Fe and NaHCO3 (Fe and Bic) in the nutrient solution for 10 d. Fe content, gene expression and enzymatic activity in different tissues were determined. A factorial statistical design with two factors (Fe and Bic) and two levels of each factor was adopted: + Fe, −Fe, + Fe + Bic and −Fe + Bic. Results indicated that bicarbonate stress increased the expression of genes MlHA1, MlFRO1 and MlIRT1 related to Fe acquisition and promoted the Fe absorption from solution. Furthermore, the presence of bicarbonate stress inhibited the expression of MlMATE66 in roots, prevented the Fe translocation from roots to developing leaf, brought about Fe accumulation in roots and reduced the Fe content in new leaf. Generally, according to our results, bicarbonate could prevent Fe translocation from roots into developing leaf, decrease Fe bioavailability and induce chlorosis in M. lupulina.






Similar content being viewed by others
Data availability
The raw data used in the present study are available within the article and Supplementary File.
References
Alhendawi RA, Römheld V, Kirkby EA, Marschner H (1997) Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum, and maize. J Plant Nutr 20:1731–1753
Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends Plant Sci 12(9):419–426
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20(1):33–40
Cao L, Yu Y, DuanMu H, Chen C, Duan X et al (2016) A novel Glycine soja homeodomain-leucine zipper (HD-Zip) I gene, Gshdz4, positively regulates bicarbonate tolerance and responds to osmotic stress in Arabidopsis. BMC Plant Biol 16(1):184
Chen Y, Barak P (1982) Iron nutrition of plants in calcareous soils. In: Brady NC (ed) Advances in Agronomy. Academic Press, pp 217–240
Connorton JM, Balk J, Rodríguez-Celma J (2017) Iron homeostasis in plants-a brief overview. Metallomics 9(7):813–823
Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000) Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 347(Pt 3):749–55
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF et al (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409(6818):346–349
Durrett TP, Gassmann W, Rogers EE (2007) The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 144(1):197–205
El-Gioushy SF, Ding Z, Bahloul AME, Gawish MS, Abou El Ghit HM et al (2021) Foliar application of nano, chelated, and conventional iron forms enhanced growth, nutritional status, fruiting aspects, and fruit quality of washington navel orange trees (Citrus sinensis L. Osbeck). Plants 10(12):2577
García MJ, García-Mateo MJ, Lucena C, Romera FJ, Rojas CL et al (2014) Hypoxia and bicarbonate could limit the expression of iron acquisition genes in Strategy I plants by affecting ethylene synthesis and signaling in different ways. Physiol Plant 150(1):95–106
Grotz N, Fox T, Connolly E, Park W, Guerinot ML et al (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci USA 95(12):7220–7224
Guo-hui Y (2012) Alkali stress induced the accumulation and secretion of organic acids in wheat. Afr J Agric Res 7
Hajiboland R, Yang X, Römheld V, Neumann G (2005) Effect of bicarbonate on elongation and distribution of organic acids in root and root zone of Zn-efficient and Zn-inefficient rice (Oryza sativa L.) genotypes. Environ Exp Bot 54:163–173
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275(3):161–203
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216(4):541–551
Hsieh EJ, Waters BM (2016) Alkaline stress and iron deficiency regulate iron uptake and riboflavin synthesis gene expression differently in root and leaf tissue: implications for iron deficiency chlorosis. J Exp Bot 67(19):5671–5685
Kruger C, Berkowitz O, Stephan UW, Hell R (2002) A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J Biol Chem 277(28):25062–25069
Lee JA, Woolhouse HW (1969) A comparative study of bicarbonate inhibition of root growth in calcicole and calcifuge grasses. New Phytol 68(1):1–11
Lescure AM, Proudhon D, Pesey H, Ragland M, Theil EC et al (1991) Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci USA 88(18):8222–8226
Liu C, Mao B, Ou S, Wang W, Liu L et al (2014) OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol Biol 84(1–2):19–36
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
López-Millán AF, Ellis DR, Grusak MA (2004) Identification and characterization of several new members of the ZIP family of metal ion transporters in Medicago truncatula. Plant Mol Biol 54(4):583–596
Lucena C, Romera FJ, Rojas CL, García MJ, Alcántara E et al (2007) Bicarbonate blocks the expression of several genes involved in the physiological responses to Fe deficiency of Strategy I plants. Funct Plant Biol 34(11):1002–1009
Martínez-Cuenca MR, Legaz F, Forner-Giner M, Primo-Millo E, Iglesias DJ (2013) Bicarbonate blocks iron translocation from cotyledons inducing iron stress responses in Citrus roots. J Plant Physiol 170(10):899–905
Mengel K (1994) Iron availability in plant tissues – iron chlorosis on calcareous soils. Plant Soil 165(2):275–283
Mengel K, Breininger MT, Bübl W (1984) Bicarbonate, the most important factor inducing iron chlorosis in vine grapes on calcareous soil. Plant Soil 81(3):333–344
Mengel K, Kirkby E, Kosegarten H, Thomas A (2001) Principles of plant nutrition. Springer Netherlands, Dordrecht, pp 585–597
Mora-Córdova CP, Tolrà R, Padilla R, Poschenrieder C, Simard M-H et al (2022) Rhizosphere acidification as the main trait characterizing the differential in vitro tolerance to iron chlorosis in interspecific pyrus hybrids (Article). Horticulturae 2022 v.8 no.6 (no. 6)
Nikolic M, Römheld V (2002) Does high bicarbonate supply to roots change availability of iron in the leaf apoplast? Plant Soil 241(1):67–74
Petit JM, Briat JF, Lobréaux S (2001) Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem J 359(Pt 3):575–582
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397(6721):694–697
Romera FJ, Alcántara E, de la Guardia MD (1997) Influence of bicarbonate and metal ions on the development of root Fe(III) reducing capacity by Fe-deficient cucumber (Cucumis sativus) plants. Physiol Plant 101:143–148
Roschzttardtz H, Conéjéro G, Divol F, Alcon C, Verdeil JL et al (2013) New insights into Fe localization in plant tissues. Front Plant Sci 4:350
Santos CS, Roriz M, Carvalho SM, Vasconcelos MW (2015) Iron partitioning at an early growth stage impacts iron deficiency responses in soybean plants (Glycine max L.). Front Plant Sci 6:325
Shahsavandi F, Eshghi S, Gharaghani A, Ghasemi-Fasaei R, Jafarinia M (2020) Effects of bicarbonate induced iron chlorosis on photosynthesis apparatus in grapevine. Sci Hortic 270:109427
Souri MK, Naiji MR, Aslani M (2018) Effect of Fe-glycine aminochelate on pod quality and iron concentrations of bean (Phaseolus vulgaris L.) under lime soil conditions. Commun Soil Sci Plant Anal 49:215–224
Takanashi K, Yokosho K, Saeki K, Sugiyama A, Sato S et al (2013) LjMATE1: a citrate transporter responsible for iron supply to the nodule infection zone of Lotus japonicus. Plant Cell Physiol 54(4):585–594
Tato L, De Nisi P, Donnini S, Zocchi G (2013) Low iron availability and phenolic metabolism in a wild plant species (Parietaria judaica L.). Plant Physiol Biochem 72:145–153
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML et al (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14(6):1223–1233
Wang J, Hou Q, Li P, Yang L, Sun X et al (2017) Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula. Plant J 90(1):79–95
Wang N, Dong X, Chen Y, Ma B, Yao C et al (2020) Direct and bicarbonate-induced iron deficiency differently affect iron translocation in kiwifruit roots. Plants (Basel) 9(11):1578
Waters BM, Blevins DG, Eide DJ (2002) Characterization of FRO1, a pea ferric-chelate reductase involved in root iron acquisition. Plant Physiol 129(1):85–94
Waters BM, Chu HH, Didonato RJ, Roberts LA, Eisley RB et al (2006) Mutations in Arabidopsis yellow stripe-like1 and yellow stripe-like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol 141(4):1446–1458
Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN et al (2007) Ethylene involvement in the regulation of the H(+)-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Biochem 45(5):293–301
Yang X, Römheld V, Marschner H (1994) Effect of bicarbonate on root growth and accumulation of organic acids in Zn-inefficient and Zn-efficient rice cultivars (Oryza sativa L.). Plant Soil 164(1):1–7
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF (2009) OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149(1):297–305
Acknowledgements
The authors would like to thank the Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province of China (U1812401) and the Science and Technology Support Plant Project of China ([2021]224) for their support in this research.
Funding
This research was supported by the Guizhou Provincial Science and Technology Projects (QIANKEHEJICHU-ZK [2023] 268), the Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province, China (U1812401), the Science and Technology Support Plant Project, China ([2021]224), Higher Education Science and Research Youth Project of Guizhou Education Department (Qianjiaoji [2022]130), the Guizhou Provincial Science and Technology Projects, China (QIANKEHEJICHU-ZK [2021] Key 038), Innovation and entrepreneurship training plan for national and provincial college students, Guizhou Normal University, China (S202110663037), Science and Technology Fund Project of Guizhou Province (No.[2020]4Y028), Guizhou forestry scientific research project, Qianlinkehe [2022] No. 28.
Author information
Authors and Affiliations
Contributions
Ximin ZHANG conceived and designed the experiments. Lunxian LIU performed the experiments on RT-PCR. Zhimeng SU performed the experiments on biomass and enzyme activity. Ming TANG designed the primers. Jing TANG identified the genes. Ximin ZHANG, Lunxian LIU, Meifeng CHEN and Xiaorong XU wrote the paper. Yin YI and Jiyi GONG revised the paper. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Communicated by V. P. Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Chen, M., Xu, X. et al. Bicarbonate affects the expression of iron acquisition and translocation genes involved in chlorosis in Medicago lupulina. Acta Physiol Plant 46, 55 (2024). https://doi.org/10.1007/s11738-024-03685-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03685-1