Abstract
α-Tocopherol (αT) has been reported to overcome the adverse effects of many environmental stresses, including salinity. A pot experiment was performed to investigate the response of salt-stressed soybean plants to the foliar application using 50, 100, or 200 mg αT L–1. The stress was applied by irrigation with three different dilutions of seawater [e.g., EC = 0.23 (tap water as a control), 3.13, or 6.25 dS m–1]. The gradual increase in salinity level gradually increased net carbon dioxide (CO2) assimilation, relative growth rate, antioxidant capacity, secondary metabolites (total phenolic compounds, flavonoids, and tannins), enzymatic activities, and DNA fragmentation due to the gradual increase in lipid peroxidation as a result of an increase in hydrogen peroxide (H2O2). On the contrary, yield components (number and weight of seeds plant–1) and photosynthetic pigment (total chlorophyll and carotenoids) contents gradually decreased with increasing salinity level. However, leaf treatment with αT, especially at 200 mg αT L‒1, preserved the highest antioxidant activities (antioxidant capacity, secondary metabolites, and enzymes), which were associated with the preservation of soybean yield and leaf pigments. These positive results occurred due to the reduction in lipid peroxidation as a result of a significant decrease in the level of H2O2. Therefore, our results recommend the use of 200 mg αT L–1 as a commercial formula for soybeans grown under saline irrigation of no more than 6.25 dS m–1.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Soybean (Glycine max L.) is a strategic crop as one of the most crucial crops for food security because the seeds are a rich source of high-quality protein and highly digestible cholesterol-free oil for human and livestock diets (Luo et al. 2005; Rady et al. 2015). The seeds contain many secondary metabolites, which have multi-health benefits as plant antioxidants (Sakthivelu et al. 2008). However, G. max is classified as a salt-sensitive or moderately salt-tolerant crop (Katerji et al. 2002).
At present, climatic changes are constantly reducing the available farmland area and its productivity due to exposure to various environmental stresses, especially salinity. Salt stress restricts plant growth due to excessive salt uptake, causing ion imbalance, hyper-osmotic stress, and “physiological drought” (Rady et al. 2015; Abd El-Mageed et al. 2020). It also overproduces oxidative stress biomarkers such as hydrogen peroxide (H2O2), superoxide radical (O2•‒), etc., which induce plant damage, including lipid peroxidation (Desoky et al. 2019; Rady et al. 2015, 2019a).
Plant metabolic pathways are salinity sensitive with a 3- to 10-fold increase in free radical production (Suzuki et al. 2012). Plants, therefore, attempt to adapt to stress through various mechanisms, including physio-biochemical responses (Negrao et al. 2017; Rady et al. 2019a), including changes in metabolism when encountering salinity (Rady et al. 2015). Secondary metabolites accumulate to participate in the control of various stresses, avoiding oxidation of essential biomolecules (Cisneros-Zevallos 2003; Kasote et al. 2015).
There are several tests to evaluate the antioxidant activity of plants (Alam et al. 2013), which have the potential to synthesize non-enzymatic antioxidants such as ascorbate (AsA), glutathione (GSH), phenolic compounds, etc. Phenolic compounds like flavonoids and tannins have promising antioxidant activity and contribute to the normal growth, development, and defense of plants (Blokhina et al. 2003; Kasote et al. 2015). The antioxidant capacity of phenolic compounds increases with the number of free hydroxyls and side chains attached to aromatic rings (Morgan et al. 1997). Therefore, they can protect plants from stress by donating electrons to guaiacol-type peroxidases to detoxify H2O2 produced excessively under stress (Sakihama et al. 2002). The antioxidant potential of phenols has always been attributed to their electron donation, ability to chelate metal ions, and low power activity (Rice-Evans et al. 1997). The flavonoids comprise phytochemicals with exceptional antioxidant activity that interact with AsA or tocopherols and synergistically intensify their biological impacts (Croft 1998). They are oxidized by peroxidase and act as scavengers of H2O2 (Michalak 2006).
Plants possess an efficient complex defense system, consisting of both enzymatic and non-enzymatic antioxidants. This system detoxifies reactive oxygen species (ROS), which are overproduced under stress conditions. The enzymatic system includes catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), peroxidase (POD), ascorbate peroxidase (APX), etc. (Chand and Dave 2009), while the non-enzymatic system consists of AsA, GSH, phenolic acids, flavonoids, tocopherols, and secondary metabolites, including tannins (Kasote et al. 2015).
To mitigate damage and increase salt tolerance in plants, potent phytochemicals (antioxidants) associated with the plant's antioxidant defense system should be used to treat foliage, including α-tocopherol (αT) (Semida et al. 2014, 2016; Rady et al. 2015, 2019b; Sadiq et al. 2019). Plants exclusively synthesize αT, which acts as a lipid-soluble redox buffer. It is generally produced in chloroplasts and protoplastids and is located in cell membranes to scavenge singlet oxygen (1O2) to protect against lipid peroxidation (Foyer 2005; Jaleel et al. 2009). It may have a complex signaling role in the pathways regulated by ROS, antioxidants, and secondary metabolites (Sadiq et al. 2019). It increases plant tolerance to salt stress by increasing antioxidant capacity, protecting polyunsaturated fatty acids from lipid peroxidation, and scavenging H2O2 (Krieger-Liszkay and Trebst 2006; Rady et al. 2011, 2015; Semida et al. 2014, 2016; Sadiq et al. 2019). αT also improves leaf photosynthetic pigments and growth in terms of net carbon assimilation rate (NAR) and relative growth rate (RGR) (Rady et al. 2015), and alters plant secondary metabolism (Mohamed and Aly 2008). In addition, it can interact with lipids and DNA to protect them from oxidative damage (Galmés et al. 2018).
Very few reports have been performed examining the protective effects of αT on secondary metabolites and genomic DNA in G. max growing under salt stress. Therefore, the main objective of the current study was to evaluate the response of growth, secondary metabolites, and antioxidant activity in salt-stressed soybean plants to the foliar application at three levels (50, 100, 200 mg L–1) of αT. This work also aimed to investigate the integrative impacts of αT and salt stress on NAR, RGR, leaf photosynthetic pigments, H2O2, malondialdehyde (MDA), enzymatic activities, and genomic DNA in soybean plants.
Materials and methods
Plant material and growth conditions
Using an open greenhouse, a pot experiment was carried out at the National Research Centre (30º 20' N; 31º 53' E), Egypt. The experiment was repeated three times over a period of time from June 6 to August 11, 2017. During this period, the average night and day temperatures were 23.1 ± 1.4 ºC and 35.5 ± 2.0 ºC, respectively, and the average relative humidity was 56 ± 6.4%.
Certified seeds of the soybean (Glycine max L. cv. “Giza 111”) were purchased from the Egyptian Agricultural Research Centre. Healthy, standardized seeds of equal size and the same color were chosen for this study. The selected seeds were cleaned using distilled water (Dw), sterilized by sodium hypochlorite (1%, v/v) for approximately 2 min, and washed again with Dw. They were left to air-dry at room temperature (25 ± 1.5 ºC) for approximately 60 min. At as equal distances as possible and at a depth of 3 cm, 10 seeds were sown in each 30 cm diameter plastic pot, containing 7.0 kg of sandy clay loam soil (Table 1). Just before sowing, commercial Bradyrhizobium japonicum granules were purchased from the Egyptian Agricultural Research (Unit of Biofertilizer Inoculum Production) and incorporated at a rate of 0.5 g pot–1 into the top 3 cm of soil.
Ammonium sulfate [20.5% N, w/w] and calcium superphosphate [15% P2O5, w/w] fertilizers were added at rates of 40 kg N and 60 kg P2O5 ha–1, respectively. Therefore, 117.6 mg N and 176.4 mg P were used per pot and incorporated into the soil just before sowing. The experiment was repeated three times, and the pots were arranged in a randomized block design. The experiment included 12 treatments; three levels of salinity (tap water and two dilutions of seawater; 3.13 and 6.25 dS m‒1) and four levels of αT (Hangzhou Toyond Biotech Co. Ltd., Zhejiang, China), which were 0 (Dw), 50, 100, and 200 mg L‒1. To prepare these concentrations, 400 mg of pure αT was dissolved in a minimal amount of methanol, then the final volume was brought to 1 L using distilled water and used as a stock (Ali et al. 2019). Each treatment included 20 pots (four replicates × five pots) and 60 plants. Seven days after sowing, three soybean seedlings were kept in each pot and irrigated with an equal volume of tap water for another 7 days based on the water-holding capacity (WHC) of the soil. Thereafter, all plants were sprayed three times, at 2, 3, and 4 weeks after sowing (WAS) with Dw, 50, 100, or 200 mg L–1 αT. The αT treatments referred to as αT0 (control), αT1, αT2, or αT3, respectively, were applied to all plants grown under three saline irrigation levels; tap water (0.23), 3.13 and 6.25 dS m‒1.
To stimulate the treatments of salt stress, seawater (EC = 51.2 dS m–1) was mixed with tap water (EC = 0.23 dS m–1; DSW0) to obtain two seawater dilutions (i.e., DSW1 with EC = 3.13 dS m–1 and DSW2 with EC = 6.25 dS m–1). Batches of plants (n = 240) were then irrigated with an equal volume of DSW with EC values of 0.23, 3.13, or 6.25 dS m–1, denoted as control (S0), S1, or S2, respectively, from 3 WAS up to 9 WAS. The EC values, pH, and concentrations of cations and anions in tap water and seawater, as well as the soil used in this experiment, are presented in Table 1. The WHC of the soil used in this study was measured by water saturation of the soil and the weight was recorded after it was drained for 48 h. The mean WHC in soil was 36% (w/v) soil: water. Soil water contents were kept at approximately 90% (w/v) of the soil WHC in all pots. The soil moisture level was controlled by weighing each pot daily and any water loss was added.
Nine WAS, two fully expanded upper fresh leaves from 24 randomly selected plants from each treatment were separated for use in different physiological and biochemical determinations, as well as for assays of enzymatic activities assays. All collected leaves were washed with Dw to remove any adherent clouds of dust. A portion of these cleaned leaves was oven-dried at 70 ºC up to constant dry weight (DW). The dried leaves were used in some determinations and another portion of leaves was left fresh for use in other determinations.
Determination of net carbon assimilation rate (NAR), relative growth rate (RGR), leaf photosynthetic pigment contents, and soybean yield
NAR and RGR were assessed at six and nine WAS using the two middle leaves of 12 plants randomly selected from each treatment. Randomly, 12 plants were carefully removed from each treatment at the ground level of pot soil and separated into leaves and stem. The leaf area was determined using a graph sheet method. Separately, the leaves and stems were oven-dried at 70 ºC until constant weight, then DW was recorded. NAR was calculated and expressed as mg DW cm‒2 using the formula of Gardner et al. (1985):
where NAR means the net CO2 assimilation rate in DW unit‒1 leaf area unit‒1 time, A means leaf area, W means total DW, and T means plant age (T1 = 6 weeks; T2 = 9 weeks). Ln A2 and ln A1; mean the values of natural logarithm-transformed leaf areas. RGR (expressed in mg mg–1 DW) was calculated using the formula of Gardner et al. (1985):
where W1 and W2 mean the plant DWs at times T1 and T2, respectively, and ln W2 and ln W1 mean the mean values of natural logarithm-transformed plant DWs.
Leaf chlorophyll and carotenoids contents were assessed using acetone extract for extraction and absorbance readings were recorded using a UV-160A UV–vis Recording Spectrometer (Shimadzu, Japan) at 663, 645, and 470 nm (Arnon 1949).
The number and weight of seeds plant‒1 were assessed at harvesting using 12 plants randomly selected from each treatment.
Quantification of total phenolic compounds, flavonoids and tannins
The contents of total phenolic compounds, flavonoids, and tannins were determined and quantified according to previous protocols (Taie et al. 2015; Taha et al. 2015).
Investigation of antioxidant activity
Antioxidant activity was determined using 1.1-diphenyl-2-picryl-hydrazil (DPPH), 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity, reducing power, iron metal chelating, and ferric reducing power (FRAP) assays according to previous studies (Abd Elkarim et al. 2020; Saleh et al. 2020).
Enzyme assays
Nine WAS, two fully expanded upper fresh leaves of plants were used immediately after cleaning with distilled water to assay enzymatic activities. Using pre-chilled mortar and pestle, 0.5 g of leaf samples (0.5 g) were homogenized in ice-cold with 100 mM P buffer (pH 7.5) containing 500 µM EDTA. The centrifuge tubes received sample homogenates to centrifuge using Beckman refrigerated centrifuge (4 °C in, 10,000 rpm, 15 min). The supernatants were used as enzymatic extracts and transferred to 30 mL tubes. Utilizing Bovine Serum Albumin V (a standard), the Bradford (1976) method was used to assess protein content in enzymatic extracts.
The activity of catalase (CAT; EC 1.11.1.6) was assayed by providing a slight modification of the Aebi (1984) method. A volume of 1.5 mL of the reaction mixture; 100 mM L‒1 phosphate buffer (pH 7.0), 0.1 mM L‒1 EDTA, 20 mM L‒1 H2O2, and 20 μL of enzymatic extract acted to start the reaction. The decrease in H2O2 was monitored at 240 nm and quantified by its molar extinction coefficient (36 M‒1 cm‒1). Results of CAT activity were expressed as Units mg‒1 protein, where 1 Unit equals 1 mM reduction of H2O2 min‒1 mg‒1 protein). The activity of ascorbate peroxidase (APX; EC 1.11.1.11) was assayed (Nakano and Asada 1981). A volume of 3 mL of the reaction mixture (50 mM potassium phosphate; pH 7.0, 0.2 mM EDTA, 0.5 mM ascorbic acid, 2% H2O2, and 0.1 mL of enzymatic extract) was used. The decrease in the absorbance reading at 290 nm was recorded for 1 min. The amount of ascorbate oxidized was calculated utilizing the extinction coefficient 2.8 mM‒1. APX was defined as 1 mM mL‒1 min‒1 at 25°C cm‒1. One unit of ascorbate oxidized as 1 mM mL‒1 ascorbate oxidized min‒1 at 25 °C. The activity of polyphenol oxidase (PPO; EC 1.10.3.1) was assayed (Kumar and Khan 1982). At 25 °C, an assaying mixture for PPO (2 mL of 0.1 M phosphate buffer; pH 6.0, 1 mL of 0.1 M catechol, and 0.5 mL of enzymatic extract) was incubated for 5 min. The reaction was then stopped by the addition of 1 mL of 2.5 N H2SO4. Absorbance readings of the formed purpurogallin were recorded at 495 nm. At zero time, 2.5 N H2SO4 was added to the blank of the same assaying mixture. The activity of PPO was expressed as Unit mg−1 protein, where the Unit equals the change in 0.1 absorbances reading min−1 mg−1 protein. The activity of peroxidase (POD; EC 1.11.1.7) was assayed (Hemeda and Klein 1990). Volumes of 25 mM L‒1 phosphate buffer (pH 7.0), 0.05% guaiacol, 10 mM L‒1 H2O2, and the enzymatic extract was the reaction mixture. The activity was evaluated by the rise in the absorbance reading at 470 nm due to the oxidation of guaiacol (E = 26.6 mM–1 cm–1). The activity of superoxide dismutase (SOD) was assayed according to Wang et al. (1983) in terms of its ability to prevent the photochemical reduction of nitro-blue-tetrazolium (NBT). One Unit of SOD activity is defined as the enzyme amount required to cause 50% inhibition of NBT auto-oxidation under the assaying conditions. SOD activity was expressed as a Unit mg‒1 protein of soybean leaf. The activity of glutathione reductase (GR; EC 1.6.4.2) was assayed (Foyer and Halliwell 1976). In a final volume of 1 mL for assaying, the medium of assaying consisted of 25 mM sodium phosphate buffer (pH 7.8), 0.5 mM oxidized glutathione (GSSG), 0.12 mM NADPH.Na4, and 0.1 mL of enzymatic extract. At 340 nm, oxidation of NADPH was observed. Utilizing the NADPH extinction coefficient (6.2 mM−1 cm−1), the enzymatic activity was calculated. One Unit of GR was defined as 1 mM mL‒1 GSSG that reduced min‒1.
Quantification of hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents
The content of H2O2 was assessed by providing a minor modification by Capaldi and Taylor (1983). Leaf samples were homogenized using TCA (5%) with 0.05 g of active charcoal at 0 °C. For 10 min, the centrifugation process was then practiced at 15,000 × g to obtain the supernatant. It was then neutralized to pH 3.6 using 4 N KOH for use in assaying H2O2. Volumes of 200 μL of leaf extract and 100 μL of 3400 µM MBTH (3-methylbenzothiazoline hydrazone) formed the reaction mixture. Initiation of the reaction was performed by adding 500 μL of the solution of horseradish peroxidase (90 Units per 0.1 L) in 0.2 M sodium acetate (pH 3.6). After 2 min, 1.4 mL of 1N HCl was added. At 630 nm, readings were recorded after 15 min.
Evaluation of MDA content indicates the level of lipid peroxidation (Madhava Rao and Sresty 2000). As an end product of lipid peroxidation, MDA content was assessed using the C4H4N2O2S (thiobarbituric acid) reaction. At 532 nm, absorbance readings were used to calculate the MDA and the measurements were corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. Calculations were performed for MDA using an extinction coefficient of 155 mM−1 cm−1.
DNA extraction and quality confirmation
Selected seed samples were ground into powder in the presence of liquid N and preserved at –20 ℃ until DNA extraction occurred. Genomic DNA extraction was performed from seed powder according to Porebski et al. (1997) with some modifications. Briefly, 200 mg of seed powder of each sample was suspended in 1 ml of extraction buffer (100 mM tris, 1.4 M NaC1, 20 mM EDTA, pH 8.0, 2% CTAB, and 0.3% β-mercaptoethanol). The mixture was incubated at 65 ℃ for 3 h with regular shaking every 30 min by inverting it several times. Then the mixtures were centrifuged at 12,000 rpm for 5 min, the supernatant was discarded and the obtained pellet was re-suspended in 1 mL of (24 chloroform: 1 isoamyl alcohol), mixed by inversion several times, and centrifuged at 15,000 rpm for 5 min. Using a clean microfuge tube, the upper aqueous phase that contained DNA was collected then, 50 μL of 7.5 M CH3COO − NH4+ (ammonium acetate) and 500 μL of ice-cold absolute ethanol were added. The solution was mixed by slowly inverting the tube several times, centrifuged at 15,000 for 2 min, and the supernatant was discarded. Then, the DNA pellet was washed with 100 µL of 70% cold ethanol and re-centrifuged at 15,000 rpm for 1 min. The supernatant was discarded and the DNA pellet was permitted to dry for 15 min at room temperature then dissolved in 100 µL of Tris–EDTA buffer and preserved at − 20 ℃ until used.
DNA quality confirmation: For evaluation of DNA quality, 10 µL of each DNA sample was electrophoresed using 1 % agarose solution stained with (0.5 µg mL−1) ethidium bromide. The run was performed at 120 v for 30 min using a TAE buffer. The gel was visualized under ultraviolet light using a gel documentation system. DNA quality was confirmed by highly resolved intact bands, indicating good DNA quality, while the smeared bands indicated degraded DNA.
All the chemicals used in this study including the solvents were of analytical grade, purchased from Sigma Chemical Co., St. Louis, MO, USA.
Statistical analysis
Experiments were arranged in a completely randomized design. Using one-way ANOVA, data were analyzed statistically, and then Duncan’s Multiple Range Test was followed to assess significance among treatments, at a 5% level of probability (p ≤ 0.05).
Results
Net assimilation rate (NAR), relative growth rate (RGR), and yield components
The salinity level of 6.25 dS m–1 significantly (p ≤ 0.05) increased the values of both NAR and RGR (by 65.6 and 24.4%, respectively), while the salinity level of 3.13 dS m–1 did not significantly increase the values of these parameters in soybean plant compared to the control (0.23 dS m–1) (Fig. 1). Foliar spray applications with αT at all rates (50, 100, or 200 mg L–1) further increased both NAR and RGR values under all salinity levels (0.23, 3.13, or 6.25 dS m–1) of the irrigation water. The maximal increases in NAR (103.5%) and RGR (60%) were achieved with the application of 200 mg αT L–1, especially under the salinity level of 6.25 dS m–1, compared to the control.
Effect of foliarly-applied αT on net carbon assimilation rate (NAR), relative growth rate (RGR), and yield components of Glycine max plant (cv. Giza 111) irrigated at different levels of salinity (0.23, 3.13, or 6.25 dS m‒1). Values are means (n = 3) ± SD. Control no saline or α-tocopherol (αT) have been applied (plants irrigated with fresh water; 0.23 dS m‒1 and sprayed with distilled water), αT1 foliar spray with 50 mg αT L‒1, αT2; foliar spray with 100 mg αT L‒1, αT3 foliar spray with 200 mg αT L‒1, S1 plants irrigated with dilute sea water (DSW) at 3.13 dS m‒1, S1 + αT1 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 50 mg αT L‒1, S1 + αT2 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 100 mg αT L‒1, S1 + αT3 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 200 mg αT L‒1, S2 plants irrigated with DSW at 6.25 dS m‒1, S2 + αT1 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 50 mg αT L‒1, S2 + αT2 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 100 mg αT L‒1, S2 + αT3 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 200 mg αT L‒1
The salinity level of 3.13 dS m–1 decreased the number and weight of seeds plant‒1 by 16.7 and 17.9%, respectively, which were further reduced by 30.0 and 50.4%, respectively, by applying the salinity level of 6.25 dS m–1 compared to the control (Fig. 1). However, αT applied at all rates (50, 100, or 200 mg L–1) increased the number and weight of seeds plant‒1 compared to the corresponding control (EC = 0.23, 3.13, or 6.25 dS m–1, respectively). The rate of 100 mg αT L–1 awarded the highest yield components (101.8 and 47.8% for the number and weight of seeds plant‒1, respectively) under the salinity level of 3.13 dS m–1. On the other hand, the highest response of these yield components was obtained with 200 mg αT L–1 under the salinity level of 6.25 dS m–1. This rate of αT increased the number and weight of seeds plant‒1 by 71.4 and 58.4% compared to the corresponding control (6.25 dS m–1).
Leaf photosynthetic pigment contents
The leaf content of chlorophyll is often evaluated to assess the effect of stress, where the development of visual symptoms and photosynthesis performance is a result of changes in the contents of leaf photosynthetic pigments. The salinity level of 3.13 dS m–1 decreased the contents of total chlorophyll, total carotenoids, and total leaf pigments by 34.5, 40.9, and 36.0%, respectively, which were further reduced by 50.3, 59.1, and 52.0%, respectively, by applying the salinity level of 6.25 dS m–1 compared to the control (Fig. 2). However, αT applied at all rates (50, 100, or 200 mg L–1) significantly increased the contents of leaf photosynthetic pigments compared to the corresponding control (0.23, 3.13, or 6.25 dS m–1, respectively). The highest response of these leaf pigments was obtained with 200 mg αT L–1 under the salinity level of 6.25 dS m–1. This αT rate elevated the contents of total chlorophyll, total carotenoids, and total leaf pigments by 64.8, 111.1, and 70.8% compared to the corresponding control (6.25 dS m–1).
Effect of foliarly-applied αT on the contents of leaf photosynthetic pigments of Glycine max plant (cv. Giza 111) irrigated at different levels of salinity (0.23, 3.13, or 6.25 dS m‒1). Values are means (n = 3) ± SD. Control no saline or α-tocopherol (αT) have been applied (plants irrigated with fresh water; 0.23 dS m‒1 and sprayed with distilled water), αT1 foliar spray with 50 mg αT L‒1, αT2 foliar spray with 100 mg αT L‒1, αT3 foliar spray with 200 mg αT L‒1, S1 plants irrigated with dilute sea water (DSW) at 3.13 dS m‒1, S1 + αT1 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 50 mg αT L‒1, S1 + αT2 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 100 mg αT L‒1, S1 + αT3 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 200 mg αT L‒1, S2 plants irrigated with DSW at 6.25 dS m‒1, S2 + αT1 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 50 mg αT L‒1, S2 + αT2 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 100 mg αT L‒1, S2 + αT3 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 200 mg αT L‒1
Antioxidant activity
Antioxidant activity in salt-stressed soybean plants was assessed by five different assays such as DPPH-radical scavenging activity, reducing power, ABTS-radical scavenging activity, metal chelating, and FRAP (Fig. 3). Approximately, all these assays showed the same trend of results. The salinity level of 3.13 dS m–1 significantly (p ≤ 0.05) increased antioxidant activity in soybean plants, which was further increased by applying the salinity level of 6.25 dS m–1 compared to the control (0.23 dS m–1). Under both salinities of 3.13 and 6.25 dS m–1, 50 mg αT L–1 caused a decrease in antioxidant activity, which tended to increase progressively with increasing αT rate, and the highest antioxidant activity was obtained with the highest αT rate (200 mg L–1).
Effect of foliarly-applied αT on antioxidant activity (assessed with five different assays) of Glycine max plant (cv. Giza 111) irrigated at different levels of salinity (0.23, 3.13, or 6.25 dS m‒1). Values are means (n = 3) ± SD. DPPH means 2,2-diphenyl, 1-picryl hydrazyl, FRAP means Ferric reducing antioxidant power, ABTS means 2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid. Control no saline or α-tocopherol (αT) have been applied (plants irrigated with fresh water; 0.23 dS m‒1 and sprayed with distilled water), αT1 foliar spray with 50 mg αT L‒1, αT2 foliar spray with 100 mg αT L‒1, αT3 foliar spray with 200 mg αT L‒1, S1 plants irrigated with dilute sea water (DSW) at 3.13 dS m‒1, S1 + αT1 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 50 mg αT L‒1, S1 + αT2 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 100 mg αT L‒1, S1 + αT3 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 200 mg αT L‒1, S2 plants irrigated with DSW at 6.25 dS m‒1, S2 + αT1 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 50 mg αT L‒1, S2 + αT2 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 100 mg αT L‒1, S2 + αT3 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 200 mg αT L‒1
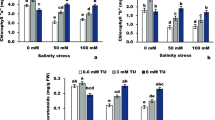
Secondary metabolites contents
The contents of secondary metabolites (e.g., total phenolic compounds, total flavonoids, and total tannins) were altered in soybean plants grown under salt stress and also changed with αT applications (Fig. 4). Compared to the control (0.23 dS m–1), the contents of all determined secondary metabolites were significantly increased with applying the salinity level of 3.13 dS m–1, while under the salinity level of 6.25 dS m–1, only tannins content was further increased. Under normal conditions, all αT rates slightly altered the contents of total phenolic compounds and total flavonoids, while 100 or 200 mg αT L–1 significantly increased the content of tannins. Under the salinity level of 3.13 dS m–1, all αT rates slightly altered the contents of all secondary metabolites, which were significantly decreased under the salinity level of 6.25 dS m–1. Maximum reductions were obtained by applying 200 mg αT L–1.
Effect of foliarly-applied αT on secondary metabolites contents in Glycine max plant (cv. Giza 111) irrigated at different levels of salinity (0.23, 3.13, or 6.25 dS m‒1). Values are means (n = 3) ± SD. Control no saline or α-tocopherol (αT) have been applied (plants irrigated with fresh water; 0.23 dS m‒1 and sprayed with distilled water), αT1 foliar spray with 50 mg αT L‒1, αT2 foliar spray with 100 mg αT L‒1, αT3 foliar spray with 200 mg αT L‒1, S1 plants irrigated with dilute sea water (DSW) at 3.13 dS m‒1, S1 + αT1 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 50 mg αT L‒1, S1 + αT2 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 100 mg αT L‒1, S1 + αT3 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 200 mg αT L‒1, S2 plants irrigated with DSW at 6.25 dS m‒1, S2 + αT1 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 50 mg αT L‒1, S2 + αT2 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 100 mg αT L‒1, S2 + αT3 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 200 mg αT L‒1b
Contents of hydrogen peroxide (H2O2) and malondialdehyde (MDA)
The content of H2O2 as a biomarker of oxidative stress and the level of MDA (an indication of lipid peroxidation) were affected by salt stress and also by foliar application with αT (Fig. 5). The salinity level of 3.13 dS m–1 increased the levels of H2O2 and MDA by 26.6 and 17.9%, respectively, and further increased by 44.2 and 51.4%, respectively, by applying the salinity level of 6.25 dS m–1 compared to the control (0.23 dS m–1). However, αT applied at all rates (50, 100, or 200 mg L–1) significantly decreased the contents of H2O2 and MDA compared to the corresponding control (0.23, 3.13, or 6.25 dS m–1, respectively). The highest reductions were obtained with 200 mg αT L–1, which reduced the contents of H2O2 and MDA by 31.7 and 55.1%, respectively, under normal conditions (0.23 dS m–1), by 32.2 and 24.1%, respectively, under the salinity level of 3.13 dS m–1, and by 31.0 and 41.0%, respectively, under the salinity level of 6.25 dS m–1.
Effect of foliarly-applied αT on the contents of the oxidative stress biomarker hydrogen peroxide (H2O2) and lipid peroxidation (in terms of malondialdehyde; MDA) of Glycine max plant (cv. Giza 111) irrigated at different levels of salinity (0.23, 3.13, or 6.25 dS m‒1). Values are means (n = 3) ± SD. Control no saline or α-tocopherol (αT) have been applied (plants irrigated with fresh water; 0.23 dS m‒1 and sprayed with distilled water), αT1 foliar spray with 50 mg αT L‒1, αT2 foliar spray with 100 mg αT L‒1, αT3 foliar spray with 200 mg αT L‒1, S1 plants irrigated with dilute sea water (DSW) at 3.13 dS m‒1, S1 + αT1 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 50 mg αT L‒1, S1 + αT2 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 100 mg αT L‒1, S1 + αT3; plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 200 mg αT L‒1, S2 plants irrigated with DSW at 6.25 dS m‒1, S2 + αT1 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 50 mg αT L‒1, S2 + αT2 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 100 mg αT L‒1, S2 + αT3 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 200 mg αT L‒1
Antioxidant enzyme activities
The activities of antioxidant enzymes such as CAT, SOD, PPO, POD, APX, and GR were altered by salt stress and αT applications (Fig. 6). The salinity level of 3.13 dS m–1 increased the enzyme activities, which increased further under the salinity level of 6.25 dS m–1 compared to the control (0.23 dS m–1). However, under both salinity levels of 3.13 and 6.25 dS m–1, αT applied at all rates (50, 100, or 200 mg L–1) significantly decreased the activities of all antioxidant enzymes, with some exceptions. The highest reductions in the activities of all enzymes were obtained with 200 mg αT L–1 under both salinity levels of 3.13 and 6.25 dS m–1. Under normal conditions, all αT rates significantly increased the activities of antioxidant enzymes, while 200 mg αT L–1 conferred the highest activities.
Effect of foliarly-applied αT on antioxidant enzyme activities in Glycine max plant (cv. Giza 111) irrigated at different levels of salinity (0.23, 3.13, or 6.25 dS m‒1). Values are means (n = 3) ± SD. CAT means catalase, SOD means superoxide dismutase, PPO means polyphenol oxidase, POD means peroxidase, APX means ascorbate peroxidase, and GR means glutathione reductase. Control no saline or α-tocopherol (αT) have been applied (plants irrigated with fresh water; 0.23 dS m‒1 and sprayed with distilled water), αT1 foliar spray with 50 mg αT L‒1, αT2 foliar spray with 100 mg αT L‒1, αT3 foliar spray with 200 mg αT L‒1, S1 plants irrigated with dilute sea water (DSW) at 3.13 dS m‒1, S1 + αT1 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 50 mg αT L‒1, S1 + αT2 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 100 mg αT L‒1, S1 + αT3 plants irrigated with DSW at 3.13 dS m‒1 and sprayed with 200 mg αT L‒1, S2 plants irrigated with DSW at 6.25 dS m‒1, S2 + αT1 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 50 mg αT L‒1, S2 + αT2 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 100 mg αT L‒1, S2 + αT3 plants irrigated with DSW at 6.25 dS m‒1 and sprayed with 200 mg αT L‒1
Genomic DNA
Figure 7 displays the agarose gel electrophoresis of genomic DNA extracted from plant tissue exposed to different degrees of salinity and exogenous αT using 1.5% agarose gel in TBE 1X buffer. The lane (M) represented a 100 bp molecular weight marker. Lanes (1) and (2) represented DNA samples of plants irrigated by tap water (0.23 dS m–1) without αT application and plants irrigated by tap water and treated with 50 mg αT L–1 and showed many DNA fragmentations. The lanes (3), (4), and (5) represented DNA with a sphere degree of DNA fragmentation, which means that the application of 100 or 200 mg αT L–1 for soybean plants showed some harmful effects and the same effect appeared at the lane of the sample from plants grown under the salinity level of 3.13 dS m‒1. On the other hand, plants grown under the salinity level of 3.13 dS m‒1 and treated with 50 or 100 mg αT L‒1 showed no DNA fragmentation, which means that αT alleviated the harmful effects of salt stress. However, plants treated with 200 mg αT L‒1 (lane 8) exhibited mild smearing in DNA. The lane (9) also represented DNA fragmentation, which indicates the harmful effect of salinity (6.25 dS m‒1) on DNA in the absence of αT. Lanes (10), (11), and (12) showed DNA enhancement, which was increased by increasing the rate of αT. The rate of 200 mg αT L‒1 (lane 12) conferred slight smearing on the gel.
Effect of foliarly-applied αT on genomic DNA in Glycine max plant (cv. Giza 111) irrigated at different levels of salinity (0.23, 3.13, or 6.25 dS m‒1). 1. Control; no saline or α-tocopherol (αT) applications have been applied (plants irrigated with fresh water; 0.23 dS m‒1 and sprayed with distilled water). 2. αT has been applied as foliar spraying at 50 mg L‒1. 3. αT has been applied as foliar spraying at 100 mg L‒1. 4. αT has been applied as foliar spraying at 200 mg L‒1. 5. Plants irrigated with dilute sea water at 3.13 dS m‒1. 6. Plants irrigated with dilute sea water at 3.13 dS m‒1 and sprayed with 50 mg αT L‒1. 7. Plants irrigated with dilute sea water at 3.13 dS m‒1 and sprayed with 100 mg αT L‒1. 8. Plants irrigated with dilute sea water at 3.13 dS m‒1 and sprayed with 200 mg αT L‒1. 9. Plants irrigated with dilute sea water at 6.25 dS m‒1. 10. Plants irrigated with dilute sea water at 6.25 dS m‒1 and sprayed with 50 mg αT L‒1. 11. Plants irrigated with dilute sea water at 6.25 dS m‒1 and sprayed with 100 mg αT L‒1. Plants irrigated with dilute sea water at 6.25 dS m‒1 and sprayed with 200 mg αT L‒1
Discussion
At present, one of the problems of climate change is the salinization of agricultural lands, especially in dry areas. To address food security challenges, tolerance strategies should be developed using exogenous antioxidants of stressed plants. Leaf treatment (foliar spray) with antioxidants is the most effective of the tolerance strategies to mitigate the harmful effects of salt stress. Foliar application of antioxidants is a promising strategy for the rapid absorption of antioxidants, passage through leaf stomata, and transport to different parts of the plant to play their beneficial roles in cellular metabolic processes (Ali et al. 2019; Rady et al. 2021; Alghamdi et al. 2023).
In the present study, salt stress-induced reductions in leaf photosynthetic pigments and soybean yield components might be due to salt-induced osmotic and oxidative stresses. Exogenously applied αT has been reported as an effective strategy to mitigate the deleterious effects of salt stress in plants (Rady et al. 2015; Semida et al. 2014, 2016; Lalarukh and Shahbaz 2018). Due to the pattern of methylation and the phenolic ring-attached three methyl groups that are able to substitute, αT has very high antioxidant activity. Therefore, accumulation of αT in plants has been reported to stimulate tolerance to abiotic stresses, including salinity in various plant species (Sharma et al. 2012). Plants have developed a wide range of reactions to overcome the potential negative effects of environmental stresses. As a major vitamin E compound, αT is located in leaf chloroplasts, specifically in their envelopes, thylakoid membranes, and plastoglobuli. αT deactivates photosynthesis-derived ROS (principally 1O2 and OH‒) and minimizes lipid peroxidation by scavenging lipid peroxyl radicals in thylakoid membranes. Depending on stress severity and species sensitivity to stress, αT levels have been shown to change differentially in response to environmental constraints (Munné-Bosch 2005).
Although Kumar et al. (2012) reported a decrease in NAR and RGR values due to a deficiency of essential nutrients in wheat plants grown under salt stress, the results obtained by Rady et al. (2015) are in agreement with the results of the current study in that the values of RGR and NAR increased in soybean plants as a result of irrigation with saline water (EC = 3.13 or 6.25 dS m–1). These increases in NAR and RGR under saline conditions may be attributed to the increased N utilization efficiency, which can be provided by the decomposition of protein molecules and/or from other N sources. Although NAR and RGR showed increased values under salt stress, the components of the soybean yield were reduced, which was preceded by the reduction of the photosynthetic pigment contents (Figs. 1, 2). These reductions may be due to the toxic impacts of salinity, which decreases or inhibits pigment biosynthesis, increases pigment degradation due to enhanced activity of chlorophyllase, and/or causes damage to chloroplast thylakoids. Additionally, salt stress has been shown to interfere with the de-novo synthesis of proteins, such as those that bind chlorophyll (Jaleel et al. 2007). However, foliar application of αT, especially at 200 mg L–1, partially restored the contents of leaf photosynthetic pigments by stimulating their biosynthesis and/or reducing their degradation due to chlorophyllase-inhibiting activity (Rady et al. 2015). Owing to the role of αT in protecting against oxidative stress and photooxidation by scavenging ROS, it maintained the integrity of photosynthetic membranes under salt stress conditions (Munné-Bosch and Alegra 2002). In addition, αT itself acts as a ROS scavenger and an integral component of membranes because it is the best antioxidant. The decreased lipid peroxidation in αT supplied soybean plants could be due to its improved content after its application and translocation into plant cells. The enhanced contents of the leaf photosynthetic pigments in salt-stressed soybean plants by exogenous αT play a crucial role in stabilizing photosynthetic processes, which improve plant metabolism. Furthermore, soybean plants supplied with αT maintained a better content of leaf carotenoids, which have a confirmed role in photosynthesis as an accessory pigment, as well as an antioxidant component, which is associated with better growth and yield as obtained in the present study. Additionally, αT plays a functional role in capturing far-red radiation to help maintain the energy balance between photosystem I (PSI) and photosystem II (PSII) (Björkman 1981), which is thus preferred for photosynthesis and C-assimilation before leaf senescence (Rady et al. 2015). As an important part of the plant defense mechanism, αT maintains the normal function and integrity of the photosynthetic apparatus (Liu et al. 2008), confirming the significant increases in leaf photosynthetic pigments by exogenously applying αT under salt stress (Fig. 2). αT directly neutralizes O2•‒ or 1O2 in plant cells (Foyer and Noctor 2005) to positively contribute to physiological processes, such as growth regulation, plant differentiation, and metabolism under saline conditions due to the increased available water and nutrients physiologically (Rady et al. 2015).
The antioxidant capacity, measured in five different assays (e.g., DPPH-radical scavenging activity, reducing power, ABTS-radical scavenging activity, metal chelating, and FRAP), was significantly increased under conditions of salt stress, and the αT application preserved the highest antioxidant activity, especially at 200 mg αT L‒1 (Fig. 3). The higher antioxidant activity, in this study, may be attributed to the increased contents of secondary metabolites (e.g., phenolic compounds, flavonoids, and tannins), which increased significantly under salt stress, and αT application maintained the highest content of secondary metabolites, especially at 200 mg αT L‒1 (Fig. 4). Under stress, higher contents of secondary metabolites contribute to a greater plant antioxidant defense due to their primary responsibility in increasing antioxidant activity. Accumulation of phenolic compounds in stressed plants is a cellular adaptive mechanism for scavenging ROS due to their potent antioxidant activities and thus preventing subcellular damage (Mohamed and Aly 2008). In their chemical structure, antioxidant action is mainly created. Phenolic compounds are oxidized by POD and contribute to H2O2 scavenging (Singh and Malik 2011). Besides, flavonoids are included in the defenses against different stresses (Winkel-Shirley 2002). They are highly effective scavengers of O2•‒, 1O2, OH‒, H2O2, etc., enabling plants to withstand different types of stress (Ali et al. 2007). The content of plant secondary metabolites is increased in stressed plants to enable them to tolerate stress (Selmar 2008). This higher antioxidant activity in salt-stressed soybean plants enriched with secondary metabolites contributed to a reduction in their H2O2 content (a biomarker of oxidative stress) and thus a decrease in their malondialdehyde (MDA; an indicator of minimal lipid peroxidation) content due to the application of αT (Fig. 5). Externally applied αT has been reported to protect lipids and other membrane components by quenching (physically) and reacting (chemically) with O2 in chloroplasts to protect PSII structure and function (Ivanov and Khorobrykh 2003). It also inhibits the chain propagation step in lipid auto-oxidation, making it a highly efficient trap for free radicals such as 1O2 (Sharma et al. 2012). As suggested in a previous study (Munné‐Bosch 2007), α‐T may influence cellular signaling by controlling the diffusion of lipid peroxidation in chloroplasts, thus modulating the formation of oxylipins that are biochemical dissection and genetic approaches have provided compelling evidence that these oxygenated derivatives are actively involved in plant defense mechanisms.
In the present study, the better performance of the soybean plant is associated with better defense mechanisms, which are of primary importance under environmental stress conditions due to plant richness in antioxidant capacity and secondary metabolites for the better functional performance of cellular membranes. Exogenously applied αT maintained better antioxidant defense mechanisms in terms of elevated activities of antioxidant enzymes (Fig. 6) and higher contents of non-enzymatic antioxidants (Fig. 4) with lower levels of H2O2 and MDA (Fig. 5). It has been suggested that αT up-regulates gene expression-encoding enzymes of the Halliwell-Asada cycle such as APX, dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR) (Li et al. 2010). αT plays an important role in regulating components of the antioxidant defense system in stressed plants. The activities of antioxidant enzymes (e.g., SOD, CAT, GR, APX, etc.) have been reported to be accelerated in response to the exogenously applied αT (Orabi and Abdelhamid 2016; Semida et al. 2016; Lalarukh and Shahbaz 2020). SOD, as the first line of defense against oxidative stress, converts O2•‒ into H2O2 (Srinieng et al. 2015). H2O2, in turn, is converted into O2 and H2O by catalases (in peroxisomes) and peroxidases (in apoplast) (Mittler 2002; Fagerstedt et al. 2010) to protect plants from oxidative damage by providing phenolic compounds for ROS elimination (Zabalza et al. 2007). These results confirm the findings of the present study that αT improved antioxidant enzyme activities and minimized the levels of H2O2 and lipid peroxidation. The present study reported the efficient roles of antioxidant enzymes in protecting soybean plants against oxidative damage. Lalarukh and Shahbaz (2020) reported further activation of POD and GR by αT to increase salt tolerance in Helianthus annuus, which is compatible with the results of the current study.
Treating soybean leaves with αT is an effective strategy for mitigating the harmful effects of salt stress on DNA (Fig. 7). αT has very important molecular advantages, such as inhibition of oxidative damage due to the scavenging capabilities of ROS and N species, and modulation of signal transduction and gene expression by several antioxidant and non-antioxidant strategies (Zingg 2007). Exogenous application of αT has been reported to scavenge the damaging impacts of salt stress in the soybean plant (Rady et al. 2015). Additionally, αT up-regulates overexpression of genes (e.g., HPT/VTE2 and TC/VTE1)-encoding enzymes in Arabidopsis for the biosynthesis of tocopherols to increase salt stress tolerance in plants to protect DNA from fragmentation against oxidative stress (ShouQiang et al. 2011; Kanwischer et al. 2005). Plants can also develop bio-defenses to mitigate harmful impacts by expressing several functions of proteins and genes in response to environmental stresses (Finatto et al. 2015; Taie et al. 2019). It has been suggested that DNA declines under salt stress due to the detrimental effect of salinity on nucleic acids (Shalata and Neumann 2001). Application of αT to salt-stressed plants may attenuate the harmful effects of salt on DNA, improve DNA biosynthesis and integrity, and/or inhibit DNA degradation by exonuclease enzymes. These enhancing effects of αT have been attributed to its reaction directly or indirectly with ROS, thus contributing to maintaining the integrity of cell proteins, lipids, and nucleic acids against damage induced by salt stress (El-Bassiouny and Sadak 2015).
Economically, the benefits to producers of applying αT to increase soybean yield under salt stress (especially 6.25 dS m–1) will be satisfactory. One hectare (through 3 αT sprays) consumes about 750 g αT (the price of about US$75.00) for about 300 000 growing plants. The average yield obtained from soybean seeds per plant is 7.3 g = 2.19-ton ha–1 = 1236 USD (according to the global soybean prices). Therefore, the net benefits are “1236 – 75” = 1161 USD ha–1. Thus, by applying 200 mg αT L–1 to soybean plants grown under salt stress (6.25 dS m–1) it will give satisfactory profits (average 1161 USD ha-1) after deducting the price of the consumed αT.
Data availability
All the data used for this experiment is given in the form of tables and figures after suitable statistical analyses.
Abbreviations
- αT:
-
α-Tocopherol
- RWC:
-
Relative growth rate
- H2O2:
-
Hydrogen peroxide
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- NAR:
-
Net CO2 assimilation rate
- Dw:
-
Distilled water
- DW:
-
Dry weight
- DSW:
-
Dilute seawater
- WHC:
-
Water-holding capacity
- DPPH:
-
1.1-Diphenyl-2-picryl-hydrazil
- APX:
-
Ascorbate peroxidase
- 1O2 :
-
Singlet oxygen
- DHAR:
-
Dehydroascorbic acid
- O2•‒ :
-
Superoxide
- AsA:
-
Ascorbic acid
- GSH:
-
Glutathione
- CAT:
-
Catalase
- GR:
-
Glutathione reductase
- MDA:
-
Malondialdehyde
- RGR:
-
Relative growth rate
- WAS:
-
Weeks after sowing
- FW:
-
Fresh weight
- S:
-
Salinity
- w/v:
-
Weight/Volume
- PPO:
-
Polyphenol oxidase
- OH‒ :
-
Hydroxyl anion
- RO:
-
Alkoxyl radicals
- MDHAR:
-
Monodehydroascorbic acid
References
Abd Elkarim AS, Ahmed AH, Taie HA, Elgamal AM, Shabana MAES (2020) Synadenium grantii hook f.: Hplc/qtof-ms/ms tentative identification of the phytoconstituents, antioxidant, antimicrobial and antibiofilm evaluation of the aerial parts. Rasayan J Chem 14(2):811–828
Abd El-Mageed TA, Rady MM, Taha RS, Abd El Azeam S, Simpson CR, Semida WM (2020) Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci Hortic 261:108930
Aebi H (1984) In: Colowick SP, Kaplan NO (Eds) Methods in enzymology, Vol. 105, Elsevier, pp. 121–126
Alam MN, Bristi NJ, Rafiquzzaman M (2013) Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 21:143–152
Alghamdi SA, Alharby HF, Bamagoos AA, Zaki SS, Abdelhamed AM, Desoky EM, Mohamed IAA, Rady MM (2023) Rebalancing nutrients, reinforcing antioxidant and osmoregulatory capacity, and improving yield quality in drought-stressed Phaseolus vulgaris by foliar application of a bee-honey solution. Plants 12(1):63
Ali RM, Kamal RK, Abbas HM (2007) The effects of treatment with polyamines on dry matter, oil and flavonoid contents in salinity stressed chamomile and sweet marjoram. Plant Soil Environ 53:529–543
Ali Q, Ali S, Iqbal N, Javed MT, Rizwan M, Khaliq R, Shahid S, Perveen R, Alamri SA, Alyemeni MN, Wijaya L, Ahmad P (2019) Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.). Sci Rep 9:12924
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris L. Plant Physiol 24:1–5
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology. Spring-Verlag, Berlin, Germany, pp 57–107
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Capaldi DJ, Taylor KE (1983) A new peroxidase color reaction: oxidative coupling of 3-methyl-2-benzothiazolinone hydrazone (MBTH) with its formaldehyde azine application to glucose and choline oxidases. Anal Biochem 129:329–336
Chand S, Dave R (2009) In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res 3:981–996
Cisneros-Zevallos L (2003) The use of controlled post-harvest abiotic stresses as a tool for enhancing the nutraceutical content and adding- value to fresh fruits and vegetables. J Food Sci 68:1560–1565
Croft KD (1998) The chemistry and biological effects of flavonoids and phenolic acids. Ann New York Acad Sci 854:435–442
Desoky EM, Merwad AMA, ElSayed AI, Rady MM (2019) Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol Biochem 142:292–302
El-Bassiouny HMS, Sadak MS (2015) Impact of foliar application of ascorbic acid and α-tocopherol on antioxidant activity and some biochemical aspects of flax cultivars under salinity stress. Acta Biol Colomb 20(2):209–222
Fagerstedt KV, Kukkola EM, Koistinen VV, Takahashi J, Marjamaa K (2010) Cell wall lignin is polymerized by class III secret able plant peroxidases in Norway spruce. J Integr Plant Biol 52(2):186–194
Finatto T, Costa de Oliveira A, Chaparro C, da Maia LC, Farias DR, Woyann LG, Mistura CC, Soares-Bresolin AP, Liauro C, Panaud O, Picault N (2015) A biotic stress and genome dynamics: specific genes and transposable elements response to iron excess in rice. Rice 8:13
Foyer CH (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling a metabolic interface between stress perception and physiological response. Plant Cell 17:1866–1875
Galmés S, Serra F, Palou A (2018) Vitamin E metabolic effects and genetic variants: a challenge for precision nutrition in obesity and associated disturbances – a review. Nutrients 10:1919
Gardner FP, Pearce RB, Mitchell RL (1985) Physiology of crop plants. Iowa State University Press, Ames, IA, USA, pp 187–208
Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55(1):184–185
Ivanov BN, Khorobrykh S (2003) Participation of photosynthetic electron transport in production and scavenging of reactive oxygen species. Antioxid Redox Sign 5(1):43–53
Jaleel CA, Manivannan P, Lakshmanan GMA (2007) NaCl as a physiological modulator of proline metabolism and antioxidant potential in Phyllanthus amarus. CR Biol 330:806–813
Jaleel CA, Riadh K, Gopi R, Manivannan P, Inès J, Al-Juburi HJ et al (2009) Antioxidant defense response: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Kanwischer M, Porfirova S, Bergmuller E et al (2005) Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol 137:713–723
Kasote DM, Katyare SS, Hegde MV, Bae H (2015) Significance of antioxidant potential of plants and its relevance to therapeutic applications – a review. Int J Biol Sci 11(8):982–991
Katerji N, Van Hoorn IW, Hamdy A, Mastrorilli M (2002) Salt tolerance classification of crops according to soil salinity and to water stress day index. In: Mediterranean crop responses to water and soil salinity: eco-physiological and agronomic analyses. Katerji N, Hamdy A, Van Hoorn IW, Mastrorilli M, (Eds). Options Méditerranéennes: Série B. Etudes et Recherches. Number 36. Bari, Italy, pp. 133–147
Krieger-Liszkay A, Trebst A (2006) Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J Exp Bot 57:1677–1684
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J Exp Biol 20:412–416
Kumar R, Singh MP, Kumar S (2012) Growth analysis of wheat (Triticum aestivum L.) genotypes under saline conditions. Int J Sci Technol Res 1:15–18
Lalarukh I, Shahbaz M (2018) Alpha-tocopherol induced modulations in morpho-physiological attributes of sunflower (Helianthus annuus) grown under saline environment. Int J Agric Biol 20:661–668
Lalarukh I, Shahbaz M (2020) Response of antioxidants and lipid peroxidation to exogenous application of alpha-tocopherol in sunflower (Helianthus annuus l.) under salt stress. Pak Jf Bot 52(1):75–83
Li Y, Zhou Y, Wang Z, Sun X, Tang K (2010) Engineering tocopherol biosynthetic pathway in Arabidopsis leaves and its effect on antioxidant metabolism. Plant Sci 178(3):312–320
Liu X, Hua X, Guo J, Qi D, Wang L, Liu Z, Jin Z, Chen S, Liu G (2008) Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotechnol Lett 30:1275–1280
Luo Q, Yu B, Liu Y (2005) Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J Plant Physiol 162:1003–1012
Madhava RKV, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J Environ Stud. 15:523–530
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mohamed AA, Aly AA (2008) Alterations of some secondary metabolites and enzymes activity by using exogenous antioxidant compound in onion plants grown under sea water stress. Am-Eur J Sci Res 3(2):139–146
Morgan JF, Klucas RV, Grayer RJ, Abian J, Becana M (1997) Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med 22:861–870
Munné-Bosch S (2005) The role of alpha-tocopherol in plant stress tolerance. J Plant Physiol 162(7):743–748
Munné-Bosch S (2007) α-Tocopherol: a multifaceted molecule in plants. Vitam Horm 76:375–392
Munné-Bosch S, Alegra L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21:31–57
Nakano Y, Asada K (1981) Hydrogen peroxide scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Negrao S, Schmockel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11
Orabi SA, Abdelhamid MT (2016) Protective role of a-tocopherol on two Vicia faba cultivars against seawater-induced lipid peroxidation by enhancing capacity of anti-oxidative system. J Saudi Soc Agric Sci 15:145–154
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15(1):8–15
Rady MM, Sadak MS, El-Bassiouny HMS, Abd Elmonem AA (2011) Alleviation the adverse effects of salinity stress in sunflower cultivars using nicotinamide and α-tocopherol. Aust J Basic Appl Sci 5:342–355
Rady MM, Sadak MSh, El-Lethy SR, Abd Elhamid EM, Abdelhamid MT (2015) Exogenous α-tocopherol has a beneficial effect on Glycine max (L.) plants irrigated with diluted seawater. J Hortic Sci Biotechnol 90(2):195–202
Rady MM, Desoky EM, Elrys AS, Boghdady MS (2019a) Can licorice root extract be used as effective natural biostimulant for salt-stressed common bean plants? S Afr J Bot 121:294–305
Rady MM, Kuşvuran A, Alharby HF, Alzahrani Y, Kuşvuran S (2019b) Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J Plant Growth Regul 38:449–462
Rady MM, Desoky EM, Ahmed SM, Majrashi A, Ali EF, Arnaout SMA, Selem E (2021) foliar nourishment with nano-selenium dioxide promotes physiology, biochemistry, antioxidant defenses, and salt tolerance in Phaseolus vulgaris. Plants 10(6):1189
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Sadiq M, Akram NA, Ashraf M, Al-Qurainy F, Ahmad P (2019) Alpha-tocopherol-induced regulation of growth and metabolism in plants under non-stress and stress conditions. J Plant Growth Regul 38:1325–1340
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicol 177:67–80
Sakthivelu G, Devi MKA, Giridhar P, Rajasekaran T, Ravishankar GA, Nikolova MT, Angelov GB, Todorova RM, Kosturkova GP (2008) Isoflavone composition, phenol content, and antioxidant activity of soybean seeds from India and Bulgaria. J Agric Food Chem 56(6):2090–2095
Saleh SA, Abd El-Galil AA, Sakr EA, Taie HA, Mostafa FA (2020) Physiochemical, kinetic and thermodynamic studies on Aspergillus wewitschiae MN056175 inulinase with extraction of prebiotic and antioxidant Cynara scolymus leaves fructo-oligosaccharides. Int J Biol Macromol 163:1026–1036
Selmar D (2008) Potential of salt stress and drought to increase pharmaceutical significant secondary compounds in plants. Landbauforschung - vTI Agric for Res 1/2(58):139–144
Semida WM, Taha RS, Abdhamid MT, Rady MM (2014) Foliar-applied alpha-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S Afr J Bot 95:24–31
Semida WM, Abd El-Mageed TA, Howladar SM, Rady MM (2016) Foliar-applied alpha-tocopherol enhances salt-tolerance in onion plants by improving antioxidant defence system. Aust J Crop Sci 10(7):1030–1039
Shalata A, Neumann P (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52(364):2207–2211
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defensemechanism in plants under stressful conditions – a review. J Bot 2012:217037
ShouQiang O, SiJie H, Peng L, WanKe Z, JinSong Z, ShouYi C (2011) The role of tocopherol cyclase in salt stress tolerance of rice (Oryza sativa). Sci China Life Sci 54(2):181–188
Singh Y, Malik CP (2011) Phenols and their antioxidant activity in Brassica juncea seedlings growing under HgCl2 stress. J Microbiol Biotechnol Res 1(4):124–130
Srinieng K, Saisavoey T, Karnchanatat A (2015) Effect of salinity stress on antioxidative enzyme activities in tomato cultured in vitro. Pak J Bot 47(1):1–10
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Taha LS, Taie HA, Hussein MM (2015) Antioxidant properties, secondary metabolites and growth as affected by application of putrescine and moringa leaves extract on jojoba plants. J Appl Pharm Sci 5(1):030–036
Taie HA, Abd-Alla HI, Ali SA, Aly HF (2015) Chemical composition and biological activities of two Solanum tuberosum cultivars grown in Egypt. Int J Pharm Pharm Sci 7(6):311–320
Taie HAA, Seif El-Yazal MA, Ahmed SMA, Rady MM (2019) Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ Sci Poll Res 26:22338–22350
Wang AG, Luo GH, Shao CB, Wu SJ, Guo JY (1983) A study on the superoxide dismutase of soybean seeds. Acta Bot Sin 9:77–84
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Zabalza A, Gaston S, Sandalio LM, Del Rio LA, Royuela M (2007) Oxidative stress is not related to the mode of action of herbicides that inhibit acetolactate synthase. Environ Exp Bot 59:150–159
Zingg J-M (2007) Vitamin E: an overview of major research directions – a review. Mol Asp Med 28(5–6):400–422
Acknowledgements
Special appreciation is extended to the National Research Centre, Dokki, Giza, Egypt for offering the facilities for this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceived, designed and performed the experiments: HAAT. Analyzed the data: HAAT. Contributed reagents/materials/analysis tools: HAAT and MMR. Wrote the paper: HAAT and MMR. All authors read and approved the final manuscript. The two co-authors participated in all stages of the work, including the conception and design of the research, the revision of the intellectual content and the drafting of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Communicated by A. Krolicka.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taie, H.A.A., Rady, M.M. α-Tocopherol mediates alleviation of salt stress effects in Glycine max through up-regulation of the antioxidant defense system and secondary metabolites. Acta Physiol Plant 46, 39 (2024). https://doi.org/10.1007/s11738-024-03654-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03654-8