Abstract
Hairy roots generated by Rhizobium rhizogenes-mediated transformation of yew (Taxus spp.) is a promising approach to enhance production of Taxol® (paclitaxel), which is one of the most effective anticancer drugs. As a prerequisite, it is pivotal to successfully produce Taxus seedlings for transformation. However, the deep dormancy of Taxus seeds leads to extreme difficulties in seed germination. Therefore, embryo rescue has been used to break the dormancy of Taxus baccata seeds, thereby producing seedlings for transformation. In the current study, a successful strategy of embryo rescue was to sterilize the surface of T. baccata seeds collected from the field at two different maturity stages (low and high). The strategy resulted in 100% germination rate, but it was worth noting that not all germinated embryos grew into fully developed plants. As a result, the present experiment introduced an innovative indicator—fully developed seedling index—to describe the growth of seedlings developed by germinated embryos. Collectively, the data revealed that 21 ± 4% of the seedlings eventually grew into fully developed plants. Regarding the development of the seedlings, the fully developed seedling index increased initially along with seedling growth, reaching a peak after 2 weeks. Subsequently, the fully developed seedling index from low maturity seeds and high maturity seeds began to decrease until it stabilized after 4 weeks and 7 weeks, respectively. Consequently, the new findings proved helpful to select T. baccata seeds with appropriate maturity, hence developing a reliable technique to produce viable seedlings for a transformation pipeline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary metabolites of several plants such as yew (Taxus spp.) can be utilized to treat cancer (Fauzee et al. 2011; Fukumoto et al. 2006), which presently is the second largest cause of mortality worldwide (Ritchie 2019). Taxol®, also known as paclitaxel, is a natural diterpenoid that has been extensively employed as a cancer chemotherapeutic drug (Schiff et al. 1979; Rowinsky and Donehower 1995; Cragg 1998). It was initially discovered in the Pacific yew tree T. brevifolia, which grows extremely slowly and contains barely approximately 0.01% (dry weight of the bark) of the chemical (Wani et al. 1971; Rowinsky and Donehower 1995; Vidensek et al. 1990). Transformation of a variety of plant species with unmodified bacterial strains of the soil bacterium Rhizobium rhizogenes (formerly Agrobacterium rhizogenes) produces distinctive hairy roots (HRs) at the site of infection, which typically result in considerable increases in beneficial secondary metabolite levels (Riker et al. 1930; Tepfer 1990; Giri and Narasu 2000; Sevon and Oksman-Caldentey 2002). Hairy root culture has recently increasingly emerged as a viable biotechnology approach for manufacturing numerous beneficial secondary metabolites (Ono and Tian 2011; Xu et al. 2008; Pellegrineschi et al. 1994). In general, R. rhizogenes has a greater success rate in infecting tissues that have a high amount of cell division or the capacity to synthesize auxin, whereas woody explants often do not have this potential (Wahby et al. 2013). A feasible technique to produce juvenile/seedling material which subsequently is used for transformation with R. rhizogenes needs to be developed. As a result, it is critical to establish and ensure high germination rate of Taxus seeds from the onset, because a high germination rate means a greater possibility of producing more seedlings.
Seed dormancy and germination are diverse physiological processes (Shu et al. 2016). An undamaged vigorous seed that cannot germinate completely under ideal circumstances is referred to as having seed dormancy (Baskin and Baskin 1998). Germination is a complicated process regulated by both internal and external stimuli (Joosen et al. 2013). Embryo development, seed coat (testa) impermeability, and phytohormones are examples of internal factors. Lulsdorf et al. (2013) reported that gibberellic acid (GA) promotes embryo growth. Similarly, application of cold or warm stratification may enhance seed dormancy release and germination. Scarification, i.e., weakening, opening, or otherwise altering the coat of a seed to promote germination, by sulfuric acid, exogenous administration of GA, and various combinations of these treatments may also accelerate seeds to break dormancy and germinate. However, the efficiency of dormancy-breaking treatments may vary by species (Baskin and Baskin 1998, 2004; Li et al. 1999; Koyuncu 2005; Vandelook et al. 2007; Tang et al. 2009; Zhang and Gusta 2010).
Embryo rescue, in which embryos are aseptically removed and cultivated in vitro, is a valuable approach for resolving seed dormancy, reducing the breeding cycle, and obtaining plants from crosses between seedless cultivars (Ho 1987; Razi et al. 2013). When seeds need a stratification interval of 2–3 months for germination in woody plants, the strategy is also suited for shortening the generation cycle (Kaur et al. 2006).
Deep dormancy of Taxus seeds is well known (Chien et al. 1998). Taxus embryos were discovered to be dormant due to two factors: embryo immaturity and the existence of endogenous inhibitors (mainly ABA) (Le Page-Degivry and Garello 1973). Separated embryos might be kept on a suitable medium under in vitro settings to solve the issue of their immaturity. To eliminate inhibitors, seeds need to be immersed in water and treated with GA, or to be immersed in water and exposed to low temperature. The importance of the maturity level of Taxus seeds as a factor influencing the germination of separated embryos was highlighted by Flores and Sgrignoli (1991), who divided the seeds into five categories based on seed maturity and argued that more mature seeds led to a lower germination rate. Chee (1994) suggested a different categorization. Three phases of development were recognized according to seed maturity. More mature seeds were discovered to be optimal for in vitro germination, showing the lack of consensus in this matter (Chee 1994).
Chee (1994) also argued for sterilizing seeds with concentrated hydrogen chloride followed by immersion in sterile water for 24 h. Zhiri et al. (1994) presented a more successful approach; in which the seeds were leached for 7 days in running tap water, then the embryos were excised and placed on a modified MS medium with activated charcoal to absorb inhibitors released by explants. The usage of charcoal resulted in a substantial enhancement with a 100% embryo germination rate. Majada et al. (2000) used a scalpel to remove the basal section of seeds, then leached the seeds under running water for 24 h, leading to a 65% germination rate. In comparison, Bian et al. (2018) obtained 90.3% germination rate without pre-treating T. yunnanensis seeds, highlighting the immense intra-species germination variability.
It is pivotal to develop a biotechnological approach to boost Taxol® content and production efficiency. The production of HRs through natural transformation of Taxus seedlings with R. rhizogenes is a highly promising strategy. Obtaining mature explants from woody plants for transformation are known since ancient times to have poor efficiency in generating HRs. In the present study, we aimed to produce viable Taxus seedlings that can be used for natural transformation to generate HRs. However, Taxus has deep seed dormancy, which results in the difficulty of direct seed germination. Thus, one purpose of present research was to break the seed dormancy of T. baccata by embryo rescue strategy. The strategy was developed for seed germination with high germination rate as a prerequisite to obtain stable and efficient seedlings generation for transformation. Another purpose of the present investigation was to explore the germination rate of T. baccata seeds with different maturity and the subsequent growth differences of seedlings, hence providing ideas for screening seeds with different maturity to obtain viable seedlings. Furthermore, the majority of studies have concentrated on overcoming the deep dormancy of Taxus seeds and optimizing the approaches to increase seed germination rate. Few studies, however, have explored the embryonic growth alterations of Taxus seeds after germination. In the present work, an innovative indicator—the fully developed seedling index—was introduced to describe the percentage of seedlings growing fully after germination. It could more accurately reflect the capacity of seeds to develop from embryonic germination to seedlings, and its development could also exhibit the growth alterations of seedlings after germination in a novel way. The importance and originality of this study is that it not only establishes an efficient embryo rescue method to break the dormancy of T. baccata seeds, thereby achieving germination, but also provides new insights into the growth of its seedlings, which contributes to more seedlings for transformation.
Materials and methods
Plant materials
The maturity stages of T. baccata seeds were categorized into the following according to the seed and aril development color (Fig. 1) (Flores and Sgrignoli 1991; Chee 1994): (1) green without aril (G, Fig. 1a); (2) green with half developing aril (GDA, Fig. 1b); (3) green with enlarging aril (EA, Fig. 1c); (4) seed with fully red aril (R, Fig. 1d). The seed in R-stage is the ultimate mature seed, which is brown when the aril is removed (Fig. 1e).
T. baccata seeds were utilized in the following two methods to break dormancy and germinate in this study:
-
(1)
Method 1 (M1)—Seeds collected from field in R-stage were directly employed for seed germination rather than embryo rescue.
-
(2)
Method 2 (M2)—Seeds were exploited for embryo rescue. These seeds came from two sources: (a) purchased from a seed company (Rarexoticseeds, Canada) (M2-C) in R-stage, which were stored for more than 1 year; (b) freshly collected from field in the same year as the experiment (M2-F) in GDA and R stages.
Pretreatment and sterilization methods
With respect to the M1 seeds, three different treatments (T1, T2, T3) were set up, each consisting of 2 independent repetitions of 35 seeds. For T1, the seeds were washed for 30 min under running tap water, then immersed in 70% (v/v) ethanol (VWR Chemicals, France) for 3 min, soaked in 2% (w/v) Ca(OCl)2 (Sigma-Aldrich, Germany) for 30 min. In T2 and T3, the seeds were killed with 65% and 96% H2SO4 (Sigma-Aldrich, Germany) for 20 min, respectively, followed by rinsing under running tap water for 3 min. Then, the seeds of T1–T3 were imbibed for 48 h in sterile water at a low temperature (4 °C). After imbibition, the seeds were submerged in 70% (v/v) ethanol for 10 min, followed by 8% (w/v) Ca(OCl)2 for 10 min. The seeds of T1–T3 were finally washed three times with sterile distilled water after treating with Ca(OCl)2.
In terms of the M2-C seeds, different pretreatment and sterilization strategies resulted in four different treatments (T4-T7), each comprising two independent repetitions of 35 embryos each. The seeds were pretreated with 96% H2SO4 for 20 min, then soaked in distilled water or GA3 (Duchefa, Netherlands) for 2 days, or leached under running tap water for 7 days. After that, the seeds were immersed in 70% (v/v) ethanol, followed by Ca(OCl)2, and lastly rinsed three times with sterile distilled water. Details of each treatment are presented in Table 1.
Regarding the M2-F seeds, two treatments were performed according to the different maturity stages, GDA (T8) and R (T9). Each treatment was composed of four independent repetitions of 35 embryos each. The arils of seeds in R-stage were removed by hand after collection. The seeds of T8 and T9 were not pretreated, and they were directly soaked in 70% (v/v) ethanol for 10 min, followed by 8% (w/v) Ca(OCl)2 for 10 min, and finally washed three times with sterile distilled water.
Seed germination
M1 seeds were directly transferred to Petri dishes containing medium A after surface sterilization and incubated at 24 ± 1 °C (ISTA 1993). The medium A was ½MS (Murashige and Skoog 1962) medium (2.2 g L–1) supplemented with 30 g L–1 sucrose (Mamone, Denmark), 1 g L–1 casein hydrolysate (Sigma-Aldrich, Germany), 1 g L–1 yeast extract (Fisher Scientific, Belgium), 0.1 g L–1 ascorbic acid (Sigma-Aldrich, Germany) and 5 g L–1 activated charcoal (Duchefa, Netherlands). The pH was adjusted to 5.7–5.8, then the medium was solidified with 2.5 g L–1 gelrite (Duchefa, Netherlands) before autoclaving for approximately 30 min at 121 °C and 105 kPa.
After 2 weeks, the seeds were transferred to culture boxes (9 cm × 8 cm × 8.5 cm, Sakata Ornamentals Europe, Marslev, Denmark) containing same medium and incubated at 24 ± 1 °C in a climate chamber (Conviron No. A1000, Canada) with 16-h photoperiod (45 μmol m –2 s –l).
In vitro culture of embryo
After surface sterilization, seeds of M2-C and M2-F were longitudinally cut in a sterile environment with help of tweezers and scalpels, the recovered embryos (Fig. 2a) were aseptically transferred to Petri dishes containing medium B (B1–B3). Details of the medium B for each treatment are shown in Table 2. The pH was adjusted to 5.7–5.8, and the medium solidified by adding 2.5 g L–1 gelrite before autoclaving for approximately 30 min at 121 °C and 105 kPa.
Embryos of each treatment were incubated in the dark at 24 ± 1 °C for 2 weeks. Then, germinated embryos were transferred to culture boxes containing the same medium and incubated at 24 ± 1 °C in a climate chamber with 16-h photoperiod (45 μmol m –2 s –l). Embryos were transferred to freshly made medium every 4 weeks.
Assessment
Seed germination was defined as radicle emergence of the seed, while embryo germination (Fig. 2b) was defined by radicle protrusion ≥ 2 mm. During the 2-week incubation period, the development status of seeds or embryos was observed every 2 days. The number of germinated seeds or embryos was recorded, and the germination rate was calculated after 2 weeks by \(\frac{\text{Germinated seeds or embryos}}{\text{Total seeds or embryos}}\)×100%
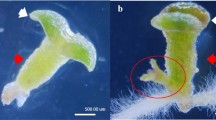
The age of seedlings from M2-F seeds was identified by weeks. After 2 weeks of dark incubation, the seedling age was recorded as 0 weeks (0 w), followed by 1 week (1 w), 2 weeks (2 w), and so on until 8 weeks (8 w). Seedlings had different stages at various ages. As shown in Fig. 3, these stages were inspired by Zarek (2007) and further developed to: (1) fully developed stage (F; seedlings were composed of straight radicle, hypocotyl and cotyledons, green and vigorous); (2) intermediate developed stage (I; seedlings had straight radicle and hypocotyl but no cotyledons, or tissue browning resulting in death); (3) abnormal developed stage (A; seedlings had curly radicle or hypocotyl). The number of seedlings in various stages was recorded every week. After that, the proportions of various stages were calculated as indexes according to the following equations:
Fully developed seedling index = \(\frac{\text{Fully developed seedlings}}{\text{Total emerged seedlings}}\)×100%, Intermediate developed seedling index = \(\frac{\text{Intermediate developed seedlings}}{\text{Total emerged seedlings}}\)×100%, Abnormal developed seedling index = \(\frac{\text{Abnormal developed seedlings}}{\text{Total emerged seedlings}}\)×100%.
During the initial 2 weeks of Petri dish incubation, each treatment was monitored for contamination every 2 days. Contaminated seeds or embryos were counted, and the contamination rate was calculated by \(\frac{\text{Contaminated seeds or embryos}}{\text{Total seeds or embryos}}\)×100%
Statistical analysis
All data were reported as mean ± standard error (SE). ANOVA was performed at α = 0.05 using SPSS software. Statistically significant differences were assumed when the p value was ≤ 0.05.
Results
Germination and contamination of seeds in each treatment
For seeds that were directly utilized for seed germination (M1), there were no germinated seeds in any treatments (Table 3). However, the contamination issue was effectively reduced by scarifying with H2SO4 in different concentrations (T2 and T3) as pretreatments compared with 100% contamination rate of T1 without pretreatment.
Regarding seeds purchased from the seed company for embryo rescue (M2-C), no germinated embryos were observed (Table 3). Nevertheless, the contamination rate of T5–T7 was dramatically decreased by 61–84% compared with T4 through increasing ethanol treatment duration and Ca(ClO)2 concentration. Among these treatments (T5–T7), the impact of leaching in running tap water for 7 days (T7) was the best, resulting in a contamination rate of less than 20%.
As can be seen from Table 3, the most significant finding was that the germination rate of embryos from seeds collected in the field at the same year as the experiments (M2-F), regardless of GDA-stage (T8) or R-stage (T9), reached 100% and there was no contamination. After 2 weeks of darkness, the embryos of both treatments appeared (Fig. 2b). As a consequence, the following results exclusively targeted the M2-F seeds.
Weekly development of seedlings from M2-F seeds
The progression of representative seedlings from R-stage seeds during 8 weeks is shown in Fig. 4, which was basically consistent with that from GDA-stage seeds (data were not shown). In general, the embryos of M2-F seeds germinated after 2 weeks in darkness (seedlings were at 0 w at this time). As illustrated in Fig. 4, the seedlings only exhibited protrusion of radicle and elongation of hypocotyl at 0 w. With the growth of hypocotyl, seedlings produced cotyledons and several new needles at 3 w. Subsequently, more new needles developed on the seedlings, and the seedlings were larger and more robust until they were at 5 w. Then the morphological changes and needle growth of seedlings began to slow down and become stable (8 w).
Variations among different stages during seedling growth
Not all germinated embryos turned into fully developed seedlings. The variations among three different stages—fully developed stage, intermediate developed stage, abnormal developed stage—of seedlings with the increase of week age are shown in Fig. 5. The stages of seedlings in the early stage of growth were variable, which indicates that seedlings were able to change from intermediate developed stage to fully developed stage, or vice versa. Even seedlings in abnormal developed stage could also change to intermediate developed stage or fully developed stage.
The change in proportion of various stages of seedlings from GDA-stage and R-stage seeds, respectively, was basically consistent (Fig. 5). The fully developed seedling index from GDA-stage and R-stage seeds started with 0%, then increased initially along with seedling growth and reached a peak at 2 w with 38 ± 2% and 43 ± 6%, respectively. After that, the fully developed seedling index from both seed stages began to decrease. Subsequently, the fully developed seedling index from GDA-stage seeds stabilized with 21 ± 3% after 4 weeks, while that from R-stage seeds did not become steady until 7 weeks with 21 ± 5%.
The intermediate developed seedling index (proportion of intermediate developed stage) from GDA-stage and R-stage seeds was 49 ± 5% and 44 ± 7%, respectively, at the beginning. Then, the intermediate developed seedling index dropped to the lowest after a week. After that, the intermediate developed seedling index from GDA-stage seeds increased rapidly within 3 weeks and reached the maximum 53 ± 3% at 4 w, and then remained steady. While the intermediate developed seedling index from R-stage seeds decreased slightly after 3 weeks of rapid growth. After that, it continued to increase and stabilized at 50 ± 4% after 7 weeks.
The abnormal developed seedling index (proportion of abnormal developed stage) from GDA-stage and R-stage seeds was 51 ± 5% and 56 ± 7% at 0 w, respectively. Subsequently, the abnormal developed seedling index from GDA-stage seeds began to decline until it reached stability with 26 ± 5% at 4 w. On the other hand, the abnormal developed seedling index from R-stage seeds decreased in the first 4 weeks and increased slightly in the following week. After that, it fell again and did not stabilize to 29 ± 5% until 7 weeks later.
There was no difference in the fully developed seedling index from GDA-stage and R-stage seeds, either at 2 w or 8 w (Fig. 5). However, the fully developed seedling index from both seeds stages at 8 w was only 21 ± 4%. It was significantly lower than the fully developed seedling index from GDA-stage (38 ± 2%) and R-stage (43 ± 6%) seeds at 2 w, which reduced by 45% and 51%, respectively.
Discussion
In vitro embryo culture
Germination rate is one of the parameters to evaluate the germination potential of seeds. Taxus seeds generally have a low natural germination rate due to deep dormancy (Le Page-Degivry and Garello 1973). Furthermore, the seed coat is impervious to water, and acid stratification has no substantial impact on the dormancy-breaking process (Thomas and Polwart 2003). At the beginning of this study, R-stage seeds collected from field were attempted to germinate directly (M1). However, they were obviously unsuccessful even when H2SO4 was employed as pretreatment (Table 3), presumably due to the deep dormancy of Taxus seeds, which was resulted from the chemical composition of endosperm. Zarek (2007) also placed seeds on the culture medium directly after collection and similarly did not observe germination, despite the addition of activated charcoal to the medium. In mature seeds, the embryo is not completely developed and is surrounded by tissues that harbors inhibitors such as ABA, making germination difficult (Le Page-Degivry and Garello 1973; Zarek 2007). Only embryos that had been separated from the surrounding endosperm and kept on an appropriate medium may germinate (Zarek 2007).
In the present study, despite the embryos of seeds purchased from seed company (M2-C) were excised for germination, no germinated embryos were detected (Table 3). This was likely due to the fact that M2-C seeds were not freshly collected and hence have been stored for a long period. Before the seeds can be utilized for germination, a certain combination of cold stratification and warm stratification was required to break seed dormancy (Chien et al. 1998). Suszka (1978) argued that germination can only occur following regularly warm and cold stratification. For Taxus seeds, a dormancy-breaking approach involving a mix of warm and cold stratification (for example, warm stratification at 22 °C for 6 months, followed by cold stratification at 5 °C for 3 months) has been developed (Devillez 1976; Suszka 1985). The stratification mode—seeds were cold stratified at 4 °C for 3 months, followed by warm stratification at 25 °C for 3 months and finally cold stratified at 4 °C for 5 months—was discovered to be more efficient by Liu et al. (2011). Yet successful, these are not practical methods to generate numerous seedlings in short time based on the lengthy procedure.
In the current experiment with respect to M2-C seeds, a successful sterilizing procedure was developed to minimize contamination, although the germination failed. The succeeding experiment employing seeds collected from field (M2-F) did not have any contamination (Table 3), which should be ascribed to the application of this sterilizing approach. Similarly, Tafreshi et al. (2011) revealed results without any contamination by soaking imbibed seeds in 70% (v/v) ethanol followed by 20 min in 5% NaClO.
Some studies reached 100% germination by leaching T. baccata seeds in running water for 7 days (Zhiri et al. 1994; Tafreshi et al. 2011). Li et al. (2019) demonstrated that hydropriming with sterile T. chinensis seeds distilled water for 3–5 days was an efficient strategy for embryo germination with 100% germination. Zarek (2007) obtained 96% germination after 28 days of cultivating the T. baccata embryos. In the current work, the germination rate of M2-F seeds of T. baccata was 100% (Table 3), which should be attributed to embryo rescue. It was formerly considered that chemical removal of the seed coat, in conjunction with seed soaking in water, was required to produce a high frequency of germination (Zarek 2007). However, our data revealed that the germination of all embryos was always 100% even when seeds were not treated with acid or soaked in water. Bian et al. (2018) also reported 90.3% germination rate with T. yunnanensis seeds without pretreatment. The procedure of embryo rescue without pretreatment in the present research further shortened the germination period of Taxus seeds.
Flores and Sgrignoli (1991) presented that greater maturity levels resulted in lower germination frequency. On the contrary, Chee (1994) proposed a conclusion that more mature seeds were better for in vitro germination. Different from their results, the findings of the current experiment demonstrated that differing maturity in same year harvested seeds were not relevant in terms of germination, and the seeds from GDA-stage and R-stage both reached 100% germination rate (Table 3). However, mature seeds purchased commercially were stored for more than 1 year and were not even responsive to embryo rescue technique (Table 3).
Growth of seedlings after embryo germination
In this study, the used seedlings were sterile because they were cultured from in vitro through embryos. Hence, they can be directly used in natural transformation experiments to produce HRs by R. rhizogenes without sterilization procedures. For this reason, the germinated embryos were not moved into soil to grow when they had roots, in order to avoid the difficulty of sterilization in subsequent experiments. Alternatively, the germinated embryos were transferred to the sterile culture boxes containing medium to develop. In respect to T. baccata seeds, however, the germinated embryos may not be able to develop completely, thus another crucial and innovative indicator was introduced in this study, which was the fully developed seedling index. It was related to T. baccata and could reflect the capacity of T. baccata seedlings to grow and maintain full development after germination. Zarek (2007) was also aware of the diverse growth responses of seedlings and classified them into 3 categories—proper germination, improper germination and callogenesis. In present study, it was worth mentioning that although the germination rate of M2-F seeds was as high as 100%, the fully developed seedling index in the later growth stage (after 7 weeks) was only 21 ± 4% (Fig. 5). This suggested that only 1/5 of the healthy seedlings might be utilized for further experiments. Flores and Sgrignoli (1991) staged that roughly 30% of embryos might eventually turned into fully developed seedlings. Another study indicated that less than 20% of the germinated embryos in T. mairei could develop fully (Chang and Yang 1996; Davarpanah et al. 2014).
In the present research, three different growth stages of T. baccata seedlings were exhibited (Fig. 5). Among the stages, the proportion of fully developed stage (fully developed seedling index) was the most noteworthy indicator. The overall development of seedlings was that the fully developed seedling index initially increased with the growth of seedlings and reached the highest value at 2 w (Fig. 5). Then, the fully developed seedling index began to decline and eventually attained stability. The fully developed seedling index at 2 w was almost twice as high as that at 8 w (Fig. 5). The decline of fully developed seedling index may be attributed to the browning induced by the lack of root development of seedlings, resulting in the reduction of fully developed seedlings (Davarpanah et al. 2014).
There was no difference between the fully developed seedling index from GDA-stage and R-stage seeds at 2 w and after stabilization (at 7 w) (Fig. 5). Accordingly, if the seedlings after 7 weeks—seedlings were larger, stronger and have more needles (Fig. 4) are to be applied for other experiments, seeds from both stages can be selected. The fully developed seedling index from GDA-stage seeds, on the other hand, gained stability at 4 w, while those from R-stage seeds remained stable at 7 w (Fig. 5). This suggested that the fully developed seedling index from R-stage seeds decreased more slowly than that from GDA-stage seeds. Consequently, if the seedlings before 7 weeks are needed, R-stage seeds could be a superior alternative compared with GDA-stage seeds.
Conclusion
In the current study, a successful strategy regarding in vitro culture of isolated embryos to break the dormancy of T. baccata seeds resulted in 100% germination rate. It is noteworthy that not all germinated embryos turned into fully developed seedlings. As a result, a promising and original indicator—the fully developed seedling index—was introduced to provide a deeper insight into seedlings developing fully after germination. Collectively, the data demonstrated that only 21 ± 4% of the seedlings could develop fully eventually. Furthermore, seed maturity had no significant influence on germination rate and ultimate fully developed seedling index in present research. The fully developed seedling index, however, from high maturity seeds decreased slower than those from low maturity after 2 weeks. In conclusion, these new findings should contribute to the selection of T. baccata seeds with appropriate maturity, hence producing seedlings. Subsequently, the seedlings will be provided for natural transformation to produce HRs, which can be further cultured to increase Taxol® production.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14(1):1–16. https://doi.org/10.1079/SSR2003150
Bian F, Su J, Liu W, Li S (2018) Dormancy release and germination of Taxus yunnanensis seeds during wet sand storage. Sci Rep 8(1):3205. https://doi.org/10.1038/s41598-018-21469-9
Chang SH, Yang JC (1996) Enhancement of plant formation from embryo cultures of Taxus mairei using suitable culture medium and PVP. Bot Bull Acad Sin 37(1):35–40
Chee PP (1994) In vitro culture of zygotic embryos of Taxus species. HortScience 29(6):695–697. https://doi.org/10.21273/HORTSCI.29.6.695
Chien CT, Kuo-Huang LL, Lin TP (1998) Changes in Ultrastructure and Abscisic Acid Level, and Response to Applied Gibberellins in Taxus mairei Seeds Treated With Warm and Cold Stratification. Ann Bot 81(1):41–47. https://doi.org/10.1006/anbo.1997.0542
Cragg GM (1998) Paclitaxel (Taxol): a success story with valuable lessons for natural product drug discovery and development. Med Res Rev 18(5):315–331. https://doi.org/10.1002/(SICI)1098-1128(199809)18:5%3C315::AID-MED3%3E3.0.CO;2-W
Davarpanah SJ, Lahouti M, Karimian R (2014) Micropropagation of common yew using embryo culture. J Appl Biotechnol Rep 1(2):77–80
Devillez F (1976) After ripening and dormancy breakdown in Taxus baccata L. seeds. In: IUFRO Proceedings of the 2nd International Symposium `Physiology of Seed Germination'. Fuji, Japan, 47±61
Fauzee NJS, Dong Z, Wang YL (2011) Taxanes: Promising anti-cancer drugs. Asian Pacific J Cancer Prev 12:837–851
Flores HE, Sgrignoli PJ (1991) In vitro culture and precocious germination of Taxus embryos. In Vitro Cell Dev Biol Plant 27(3):139–142. https://doi.org/10.1007/BF02632197
Fukumoto S, Sawasaki E, Okuyama S, Miyake Y, Yokogoshi H (2006) Flavor components of monoterpenes in citrus essential oils enhance the release of monoamines from rat brain slices. Nutr Neurosci 9:73–80. https://doi.org/10.1080/10284150600573660
Giri A, Narasu ML (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18(1):1–22. https://doi.org/10.1016/S0734-9750(99)00016-6
Ho RH (1987) Embryo Culture. Cell and Tissue Culture in Forestry. 2. In: "Specific Principles and Methods: Growth and Development", (Eds.): Bonga JM and Durzan DJ. Martinus Nijhoff Publishers, Dordercht, pp 137–167
ISTA [International Seed Testing Association]. 1993. International rules for seed testing: rules 1993. Seed Science and Technology 21 (Suppl.): 1–259
Joosen RVL, Arends D, Li Y, Willems LAJ, Keurentjes JJB, Ligterink W, Jansen RC, Hilhorst HWM (2013) Identifying genotype-by-environment interactions in the metabolism of germinating Arabidopsis seeds using generalized genetical genomics. Plant Physiol 162(2):553–566. https://doi.org/10.1104/pp.113.216176
Kaur R, Sharma N, Kumar K, Sharma DR, Sharma SD (2006) In vitro germination of walnut (Juglans regia L.) embryos. Sci Hortic 109(4):385–388. https://doi.org/10.1016/j.scienta.2006.05.012
Koyuncu F (2005) Breaking seed dormancy in black mulberry (Morus nigra L.) by cold stratification and exogenous application of gibberellic acid. Acta Biol Crac Ser Bot 47(2):23–26
Le Page-Degivry MT, Garello G (1973) Embryo dormancy in Taxus baccata: influence of culture medium on initiation of germination. Physiol Plant 29:204–207. https://doi.org/10.1111/j.1399-3054.1973.tb03093.x
Li X, Baskin JM, Baskin CC (1999) Anatomy of two mechanisms of breaking physical dormancy by experimental treatments in seeds of two North American Rhus species (Anacardiaceae). Am J Bot 86(11):1505–1511. https://doi.org/10.2307/2656788
Li YL, Yang Q, Yu XY, Wang L, Wang WX, Xiong XY (2019) Induction of Half-Sib Embryonic Callus and Production of Taxiod Compounds from Taxus chinensis var. mairei. Int J Agric Biol 21(4):719–725. https://doi.org/10.17957/IJAB/15.0949
Liu D, Yu HL, Li FL, Guo HH (2011) An analysis of dormancy and dormancy release in Taxus chinensis var. mairei seeds. Seed Sci Technol 39(1):29–43. https://doi.org/10.15258/sst.2011.39.1.04
Lulsdorf MM, Yuan HY, Slater SM, Vandenberg A, Han X, Zaharia LI, Abrams SR (2013) Endogenous hormone profiles during early seed development of C. arietinum and C. anatolicum. Plant Growth Regul 71(2):191–198. https://doi.org/10.1007/s10725-013-9819-2
Majada JP, Sierra MI, Sanchez-Tames R (2000) One step more towards taxane production through enhanced Taxus propagation. Plant Cell Rep 19(8):825–830. https://doi.org/10.1007/s002990000196
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ono NN, Tian L (2011) The multiplicity of hairy root cultures: prolific possibilities. Plant Sci 180(3):439–446. https://doi.org/10.1016/j.plantsci.2010.11.012
Pellegrineschi A, Damon JP, Valtorta N, Paillard N, Tapfer D (1994) Improvement of ornamental characters and fragrance production in lemon-scented geranium through genetic-transformation by Agrobacterium rhizogenes. Nat Biotechnol 12:64–68. https://doi.org/10.1038/nbt0194-64
Razi M, Marandi RJ, Baneh HD, Hosseini B, Darvishzadeh R (2013) Effect of Paternal Genotypes Sprays with BA and IAA Concentration on Embryo Rescue of F1 Progenies from'Askari'(Vitis vinifera L.) Cultivar. J Agr Sci Tech 15(5):1023–1031
Riker AJ, Banfield WM, Wright WH, Keitt GW, Sagen HE (1930) Studies on infectious hairy root of nursery apple trees. J Agric Res 41(7):507–540
Ritchie H (2019) what-does-the-world-die-from@ourworldindata.org (WWW Document). Our World Data. URL https://ourworldindata.org/what-does-the-world-die-from (accessed 12.17.20)
Rowinsky EK, Donehower RC (1995) Paclitaxel (Taxol). N Engl J Med 332:1004–1014. https://doi.org/10.1056/NEJM199504133321507
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667. https://doi.org/10.1038/277665a0
Sevon N, Oksman-Caldentey KM (2002) Agrobacterium rhizogenes-mediated transformation: root cultures as a source of alkaloids. Planta Med 68(10):859–868. https://doi.org/10.1055/s-2002-34924
Shu K, Liu XD, Xie Q, He ZH (2016) Two faces of one seed: hormonal regulation of dormancy and germination. Mol Plant 9(1):34–45. https://doi.org/10.1016/j.molp.2015.08.010
Suszka B (1978) Generative and vegetative reproduction. In: Bartkowiak S, Bugala W, Czartoryski A, Hejnowicz A, Król S, Srodon A, Szaniawski RK (eds) The yew—Taxus baccata L. National Technical Information Service, Washington, DC, pp 87–102
Suszka B (1985) Conditions for after-ripening and germination of seeds and for seedling emergence of English yew (Taxus baccata L.). Arbor Kornickie 30:285–338
Tafreshi SAH, Shariati M, Mofid MR, Nekui MK (2011) Rapid germination and development of Taxus baccata L. by in vitro embryo culture and hydroponic growth of seedlings. In Vitro Cell Dev Biol Plant 47(5):561–568. https://doi.org/10.1007/s11627-011-9369-0
Tang AJ, Tian MH, Long CL (2009) Seed dormancy and germination of three herbaceous perennial desert ephemerals from the Junggar Basin. China Seed Sci Res 19(3):183–189. https://doi.org/10.1017/S096025850999002X
Tepfer D (1990) Genetic transformation using Agrobacterium rhizogenes. Physiol Plant 79(1):140–146. https://doi.org/10.1111/j.1399-3054.1990.tb05876.x
Thomas PA, Polwart A (2003) Taxus baccata L. Biological flora of the British isles. J Ecol 91(3):489–524.
Vandelook F, Bolle N, Van Assche JA (2007) Seed dormancy and germination of the European Chaerophyllum temulum (Apiaceae), a member of a trans-Atlantic genus. Ann Bot 100(2):233–239. https://doi.org/10.1093/aob/mcm090
Vidensek N, Lim P, Campbell A, Carlson C (1990) Taxol content in bark, wood, root, leaf, twig, and seedling from several Taxus species. J Nat Prod 53:1609–1610. https://doi.org/10.1021/np50072a039
Wahby I, Caba JM, Ligero F (2013) Agrobacterium infection of hemp (Cannabis sativa L.): establishment of hairy root cultures. J Plant Interact 8(4):312–320. https://doi.org/10.1080/17429145.2012.746399
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93(9):2325–2327. https://doi.org/10.1021/ja00738a045
Xu H, Kim YK, Suh SY, Uddin MR, Lee SY, Park SU (2008) Decursin production from hairy root culture of Angelica gigas. J Korean Soc Appl Biol Chem 51(4):349–351. https://doi.org/10.3839/jksabc.2008.062
Zarek M (2007) A practical method for overcoming the dormancy of Taxus baccata isolated embryos under In vitro conditions. In Vitro Cell Dev Biol Plant 43:623–630. https://doi.org/10.1007/s11627-007-9064-3
Zhang W, Gusta LV (2010) Germination response of black and yellow seed coated canola (Brassica napus) lines to chemical treatments under cold temperature conditions. Plant Growth Regul 60(2):105–114. https://sci-hub.wf/https://doi.org/10.1007/s10725-009-9425-5
Zhiri A, Jaziri M, Homes J, Vanhaelen M, Shimomura K (1994) Factors affecting the in vitro rapid germination of Taxus embryos and the evaluation of taxol content in the plantlets. Plant Cell Tiss Organ Cult 39(3):261–263. https://sci-hub.wf/https://doi.org/10.1007/BF00035979
Funding
Open access funding provided by Royal Library, Copenhagen University Library. JH reports financial support was provided by China Scholarship Council (PhD grant no. 201906760024). XC reports financial support was provided by China Scholarship Council (PhD grant no. 202106850009). BTF and HL report financial support was provided by Independent Research Fund Denmark Technology and Production (grant no. 0136-00410B).
Author information
Authors and Affiliations
Contributions
JH: data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing. BTF: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, supervision, visualization, writing—review and editing. XC: investigation, methodology, writing—review and editing. RM: conceptualization, methodology, supervision, writing—review and editing. HL: conceptualization, formal analysis, funding acquisition, investigation, methodology, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Gniazdowska-Piekarska.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, J., Favero, B.T., Chen, X. et al. Overcoming seed dormancy of Taxus baccata L. by embryo rescue leads to germination and seedling growth. Acta Physiol Plant 45, 127 (2023). https://doi.org/10.1007/s11738-023-03611-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-023-03611-x