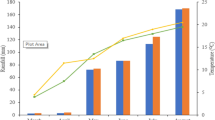
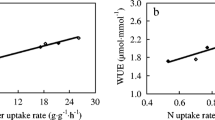
Abstract
Water and nitrogen (N) fertilizer are the two main factors affecting wheat growth and yield. Spring wheat cultivars, Spitfire (drought sensitive) and Drysdale (drought tolerant), were used as materials for studying N metabolism physiological and molecular dynamics under water-deficit treatment at high-N level (180 kg hm−2, i.e., 80 mg kg−1) vs. low-N level (22.5 kg hm−2, i.e., 10 mg kg−1) at heading stage in this experiment. The results showed that the chlorophyll, soluble sugar, soluble protein and free amino acid contents; glutamine synthetase (GS), glutamate synthetase (GOGAT) and phosphoenolpyruvate carboxylase (PEPC) enzyme activities; gene GS1 expression; and grain yield were increased at high-N level compared to low-N level under water-deficient stress at heading stage in both cultivars. Relative expressions of genes GDH, GOGAT and PEPC were down-regulated in Spitfire under water-deficit treatment, but were up-regulated in Drysdale. The indicators of root system architecture, including root surface area, total root volume, root diameter and number of root tips and root branches, were increased at high-N level under water-deficient treatment in both cultivars, whereas total root length decreased. The root–shoot ratio of both cultivars decreased to low-N level under water-deficit treatment. The N transfer rate was significantly increased at high-N level after heading for water-deficit treatment. The grain yields of both cultivars were maintained by the high-N level under water-deficit treatment. Our results suggested a high-N level could alleviate the damage from water deficiency by activating genes/enzymes related to wheat carbon and N metabolism.






Similar content being viewed by others
References
Abdelkader A, Aronsson H, Sundqvist C (2007) High salt-stress in wheat leaves (Triticum aestivum L.) causes retardation of chlorophyll accumulation due to a limited rate of protochlorophyllide formation. Physiol Plant 130:157–166
Abid M, Tian Z, Ata-Ul-Karim S, Cui Y, Liu Y, Zahoor R, Jiang D, Dai T (2016) Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods. Front Plant Sci 7:981. https://doi.org/10.3389/fpls.2016.00981
Agami R, Ghramh H, Hashem M (2017) Seed inoculation with Azospirillum lipoferum alleviates the adverse effects of drought stress on wheat plants. J Appl Bot Food Qual 90:165–173
Agami R, Alamri S, Abd El-Mageed T, Abousekken M, Hashem M (2018) Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agri Water Manage 210:261–270
Ata-Ul-Karim S, Liu X, Lu Z, Yuan Z, Zhu Y, Cao W (2016) In-season estimation of rice grain yield using critical nitrogen dilution curve. Field Crops Res 195:1–8
Atkin O, Macherel D (2009) The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot 103:581–597
Balkos K, Britto D, Kronzucker H (2010) Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L.Cv.IR-72). Plant Cell Environ 33:23–34
Barash I, Sadon T, Mor H (1973) Induction of a specific isoenzyme of glutamate dehydrogenase by ammonia in oat leaves. Nat New Biol 244:150–152. https://doi.org/10.1038/newbio244150a0
Barbottin A, Lecompte C, Bouchard C, Jeuffroy M (2005) Nitrogen remobilization during grain-fill in wheat: genotypic and environmental effects. Crop Sci 45:1141–1150
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annal Biochem 72:248–254
Brueck H, Erdle K, Gao Y, Giese M, Zhao Y, Peth S, Lin S (2010) Effects of N and water supply on water use-efficiency of semiarid grassland in inner Mongolia. Plant Soil 328:495–505
Comas L, Eissenstat D, Lansko A (2000) Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol 147:171–178
Dowla N, Edwards I, O’Hara G, Islam S, Ma W (2018) Developing wheat for improved yield and adaptation under a changing climate: optimization of a few key genes. Engineering 4:514–522
Fales F (1951) The assimilation and degradation of carbohydrates by yeast cells. J Bio Chem 193:113–124
Foyer C, Noctor G, Hodges M (2011) Respiration and nitrogen assimilation: targeting mitochondria-associated metabolism as a means to enhance nitrogen use efficiency. J Exp Bot 62:1467–1482
Gaju O, Allard V, Martre P, Snape J, Heumez E, Le GJ, Moreau D, Bogard M, Griffiths S, Orford S, Hubbart S, Foulkes M (2011) Identification of traits to improve the nitrogen-use efficiency of wheat genotypes. Field Crops Res 123:139–152
Gonza´lez M, Sanchez R, Cejudo F (2003) Abiotic stresses affecting water balance induce phosphoenolpyruvate carboxylase expression in roots of wheat seedlings. Planta 216:985–992
Goodall A, Kumar P, Tobin A (2013) Identification and expression analyses of cytosolic glutamine synthetase genes in barley (Hordeum vulgare L.). Plant Cell Physiol 54:492–505
Grabowska A, Nowicki M, Kwinta J (2011) Glutamate dehydrogenase of the germinating triticale seeds: gene expression, activity distribution and kinetic characteristics. Acta Physiol Plant 33(5):1981–1990
Hoai N, Shim I, Email A, Kobayashi K, Kenji U (2003) Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 41:159–164
Hochman Z, Horan H (2018) Causes of wheat yield gaps and opportunities to advance the water-limited yield frontier in Australia. Field Crops Res 228:20–30
Jogaiah S, Govind S, Tran L (2013) Systems biology-based approaches toward understanding drought tolerance in food crops. Crit Rev Biotech 33:23–39
Khan N, Zandi P, Ali S, Mehmood A, Adnan S (2018) Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front Microbiol 9:2507
Lalelou F, Fateh M (2014) Effects of water deficit stress and nitrogen fertilizer on wheat varieties. Int J Biosci 4:183–189
Lawlor D (2002) Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and role of ATP. Ann Bot 89:871–885
Livak J, Schmittgen T (2001) Analysis of relative gene expression aata using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 25:402–408
Manavalan L, Guttikonda S, Nguyen V, Shannon J, Nguyen H (2010) Evaluation of diverse soybean germplasm for root growth and architecture. Plant Soil 330:503–514
Manschadi A, Christopher J, DeVoil P, Hammer G (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33:823–837
Manschadi A, Hammer G, Christopher J, de Voil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129
Masclaux-Daubresse C, Reisdorf-Cren M, Pageau K et al (2006) Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol 14(2):444–456. https://doi.org/10.1104/pp.105.071910
Mobasser H, Mohammadi G, Heidari H, Abad S, Rigi K (2014) Effect of application elements, drought stress and variety on nutrients of grain wheat in Zahak region. J Biodivers Environ Sci 5:105–110
Moore S, Stein W (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211:907–913
Nagy Z, Nemeth E, Guoth A, Bona I, Wodala B, Pescvaradi A (2013) Metabolic indicators of drought stress tolerance in wheat: glutamine synthetase isoenzymes and Rubisco. Plant Physiol Biochem 67:48–54
Narayanan S, Mohan A, Gill K, Vara Prasad P (2014) Variability of root traits in spring wheat germplasm. PLoS One 9:0100317. https://doi.org/10.1371/journal.pone.0100317
Osuna L, Gonza´lez M, Cejudo F, Vidal J, Echevarrı´a C (1996) In vivo and in vitro phosphorylation of the phosphoenolpyruvate carboxylase from wheat seeds during germination. Plant Physiol 111:551–558
Peterhansel C, Maurino V, Manavalan L, Guttikonda S, Nguyen V, Shannon J, Nguyen H (2010) Evaluation of diverse soybean germplasm for root growth and architecture. Plant Soil 330:503–514
Rastegari A, Yadav AN, Awasthi AA (2020) Yadav N (2020) Trends of microbial biotechnology for sustainable agriculture and biomedicine systems: diversity and functional perspectives. Elsevier, Amsterdam, The Netherlands
Richardson A, Duigan S, Berlyn G (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194
Shamsi K (2010) The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J Anim Plant Sci 8:1051–1060
Singh M, Singh V, Prasad S (2016) Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physio Biochem 109:72–83
Skopelitis D, Paranychianakis N, Paschalidis K, Pliakonis E, Delis I, Yakoumakis D, Kouvarakis A, Papadakis A, Stephanou E, Roubelakis-Angelakis K (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18:2767–2781
Stewart G, Rhodes D (1978) Nitrogen metabolism of halophytes III enzymes of ammonia assimilation. New Phyto 80:307–316
Stoscheck C (1990) Quantitation of protein. Methods Enzymol 182:50–68
Tercé-Laforgue T, Bedu M, Dargel-Grafin C, Dubois F, Gibon Y, Restivo F, Hirel B (2013) Resolving the role of plant glutamate dehydrogenase: II. Physiological characterization of plants overexpressing the two enzyme subunits individually or simultaneously. Plant Cell Physiol 54:1635–1647
Vanlerberghe G, Schuller K, Smith R, Feil R, Plaxton W, Turpin D (1990) Relationship between NH4+ assimilation rate and in Vivo phosphoenolpyruvate carboxylase activity. Plant Physiol 94:284–290
Wu F, Bao W, Li F, Wu N (2008) Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Env Exp Bot 63:248–255
Xu Z, Zhou GS (2006) Nitrogen metabolism and photosynthesis in Leymus chinensis in response to long-term soil drought. J Plant Growth Regul 25:252–266
Yang J, Zhang J (2006) Grain filling of cereals under soil drying. New Phytol 169:223–236
Yao Y, Lv L, Zhang L, Yao H, Dong Z, Zhang J, Ji J, Jia X, Wang H (2019) Genetic gains in grain yield and physiological traits of winter wheat in Hebei Province of China, from 1964 to 2007. Field Crops Res 239:114–123
Yemm E, Willis A (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Zhang L, Li S, Zhang H, Liang ZS (2007) Nitrogen rates and drought stress effect on production, lipid peroxidation and antioxidative enzyme activities in two maize (Zea mays L.) genotypes. J Agro Crop Sci 193:387–397
Zhang L, Chu Q, Jiang Y, Chen F, Lei Y (2021) Impacts of climate change on drought risk of winter wheat in the North China Plain. J of Inte Agri 20(10):2601–2612
Zhong C, Cao X, Hu J, Zhu L, Zhang J, Huang J, Jin Q (2017) Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front Plant Sci 8:1079
Zhong C, Cao X, Bai Z, Zhang J, Zhu L, Huang J, Jin Q (2018) Nitrogen metabolism correlates with the acclimation of photosynthesis to short-term water stress in rice (Oryza sativa L.). Plant Physiol Biochem 125:52–63
Zhong C, Bai Z, Zhu L, Zhang J, Zhu C, Huang J, Jin Q, Cao X (2019) Nitrogen-mediated alleviation of photosynthetic inhibition under moderate water deficit stress in rice (Oryza sativa L.). Env and Exp Bot 157:269–282
Zhu J, Ingram P, Benfey P, Elich T (2011) From lab to field, new approaches to phenotyping root system architecture. Curr Opin Plant Biol 14:310–317
Zodape S, Gupta A, Bhandari S, Rawat U, Chaudhry D, Eswaran K, Chikara J (2011) Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J Sci Ind Res 70:215–219
Acknowledgements
This work was supported by the Key Research Foundation of Science and Technology Department of Zhejiang Province (2021C02064-5) and the project of improving nitrogen use efficiency in wheat from the Sino-Australia Joint Center for Wheat Improvement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, M., Jiang, Y., Liu, L. et al. Benefits of high nitrogen fertilizer on nitrogen metabolism, nitrogen transfer rate, root system architecture and grain yield of wheat (Triticum aestivum L.) under water deficit at heading stage. Acta Physiol Plant 44, 121 (2022). https://doi.org/10.1007/s11738-022-03460-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03460-0