Abstract
In this study, two poplar varieties with different resistance to sulfur dioxide were selected for a comparative experiment. SO2 fumigation to the poplars was carried out under controlled conditions to determine the variation in the activity of enzymes, the content of metabolites and the expression of enzymes genes in pathways of sulfur metabolism in plants. The results showed that the activity of enzymes and the content of sulfur metabolites were closely related to the response of the poplars to SO2 stress. Populus × euramericana cv. 'Purui' had two ways of detoxification: oxidation detoxification, oxidizing sulfite (SO32−) to sulfate SO42− by sulfite oxidase; reductive detoxification, SO32− being reduced to S2− by sulfite reductase (SiR). Moreover, OASTL and SAT activity, and levels of cysteine (CYS) and glutathione (GSH) also increased in P. × euramericana cv. 'Purui' in response to SO2 fumigation, and the gene expression encoding Glutathione S-transferases (GST), and some enzymes in cysteine and methionine metabolism was up-regulated. For Populus × euramericana cv. '74/76' with weaker resistance to sulfur dioxide, it only detoxified by increasing the activity of SiR, and but down-regulated the expression of gene encoding 3'-phosphoadenosine 5'-phosphosulfate synthase (PAPSS), which could affect the consumption of sulfite in the exposure to SO2. Thus, the SO2-resistant difference of the two poplar varieties is mainly attributed to variation in activity of the enzymes and content of their metabolites in pathways of sulfur metabolism, and gene expression of some enzymes in cysteine and methionine metabolism also plays a role in the resistant difference.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfur dioxide (SO2) is one of the hazardous environmental pollutant gases and mainly emitted from the combustion of fossil fuel and eruption of volcanoes. SO2 enters plant leaves mainly through stomata. On the cell surface or in the cytoplasm, SO2 is converted into bisulfite and sulfite ions after encountering water. These ions can lead to the production of free radical ions such as reactive oxygen species (ROS) in plants (Asada and Kiso 1975; Alscher 1984). They initiate a series of reactions in plants, leading to metabolic changes. Some of these changes will cause serious harm to plants. High concentration of SO2 may even lead to acute leaf necrosis or even plant death. To maintain physiological activity and survival, plants have evolved effective protective mechanisms against sulfur dioxide stress (Kondo 2002). These bisulfite and sulfite ions enter the sulfur assimilation process in plants, during which they are reduced and converted to cysteine and other sulfur compounds (Filner et al. 1984; Heber and Hüve 1998), or oxidized to sulfate (Pfanz et al. 1990; Eilers et al. 2001).
In peroxisome, sulfite oxidase (so) catalyzes the conversion of sulfite (so32−) to sulfate (so42−) (Rennenberg and Herschbach 2014, refer there). The generated sulfate can be stored in vacuoles or directly entered the assimilation pathway. In chloroplast or cytoplasm, sulfate is activated by ATP sulfonylase (ATPS) to form adenosine 5´-phosphate sulfate (APS). APS can then be further phosphorylated to 3'-adenosine phosphate 5'-phosphate sulfate (PAPS) by APS kinase (APK) or reduced to sulfite (so32−) by APS reductase (APR). On the other hand, sulfite can be reduced to sulfide (s2−) by sulfite reductase (SIR). In chloroplasts, mitochondria, and cytoplasm, sulfide combines with o-acetylserine (OAS) to form cysteine (Cys), which is the precursor of all sulfur-containing organic compounds. Serine acetyltransferase (SAT) synthesizes o-acetylserine (OAS), a precursor of Cys, and then o-acetylserine (mercaptan) lyase (oastl) exchanges acetate of OAS for sulfide. Cysteine is mainly used to synthesize proteins, In addition to protein synthesis, glutathione (GSH) is considered to be the main product of cysteine consumption.
If sulfites persist in plant cells for a long time, plants will suffer serious stress and damage. The key to detoxification is to convert bisulfite and sulfite as soon as possible. A previous study has found that sulfite oxidation and sulfite reduction as well as the assimilation all contribute to SO2 detoxification of Poplar that is exposed to SO2 (Randewig et al. 2014). Although the detoxification mechanisms of plants to sulfite are clear, whether these attributes are related to the ability of plants to resist sulfur dioxide has not been reported. As we know there are different resistances to SO2 over various plant species. Is the resistance to sulfur dioxide reflected in these attributes? In addition, SO2 evokes a comprehensive reprogramming of metabolic pathways, and there are significant changes in the transcript abundance of genes that participate in SO2 metabolic pathways in Arabidopsis (Zhao and Yi 2014). The transcriptome analysis of poplars under SO2 fumigation is not yet clarified.
Before 2017, China was the largest emitter of sulfur dioxide in the world due to the rapid development of industrialization (Li 2017). In a previous study, we found that the poplar variety of Populus × euramericana cv. 'Purui' expressed stronger resistance to SO2 contamination compared with another similar poplar variety of Populus × euramericana cv. '74/76' (Xu et al. 2011). P. × euramericana cv. 'Purui' has some characteristics of coping with SO2 stress compared with P. × euramericana cv. '74/76' as follows: 1) maintains relatively higher net assimilation rate after fumigation for 2 h with 9.1 μL L−1 SO2 fumigation; 2) has higher reduced glutathione (GSH) content and superoxide dismutase (SOD) activity; 3) has thicker cuticle, and larger size of stomata with a lower density (Xu et al. 2011). The cuticle can function as the first barrier for toxic gases (Tamm and Cowling 1977), but sulfur dioxide intake into leaves through cuticle cannot be fully neglected, although it is a proceeding at low rates (Lendzian 1984). As mentioned above, SO2 enters plants mainly through stomata; thus, stomatal density and aperture are important factors that determine SO2 entering plants. When subjected to SO2 stress, some plants avoid excessive toxic gas inhalation through sensitive stomatal movement (Robinson et al. 1998; Grulke et al. 2007). However, it is not clear whether the variety brings into play in its resistance to SO2 pollution by suppressing the entrance of the pollutant or reducing the toxic of SO2 in leaves. In a preliminary experiment, we have found that there is more sulfate content in P. × euramericana cv. 'Purui' leaves compared with P. × euramericana cv. '74/76' in the environment with mild SO2 pollution, suggesting that P. × euramericana cv. 'Purui' may not suppress the entrance of SO2 into the leaves. To further study the resistant mechanism of P. × euramericana cv. 'Purui' to SO2 pollution, in this study, we compared the differences in the detoxification mechanism, including effects of SO2 on leaf enzyme activities of the sulfate assimilation pathway, sulfur metabolites, and transcriptional regulation of enzymes related to sulfur metabolism, between P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76'. Under SO2 fumigation, the co-regulation of enzymes involved in the sulfur assimilation pathway and the impact on the transcript levels of genes involved in the sulfur metabolite, cysteine and methionine metabolite, and glutathione metabolite were investigated in P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' varieties. The main aim of the present study is to identify the differences in detoxification mechanism and gene expression changes of sulfur metabolism between P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76', and whether these features are related to the ability of the poplars to resist sulfur dioxide.
Materials and methods
Plant material
This experiment was performed with 1-year-old cuttings of two poplar varieties, Populus × euramericana CV. ‘Purui’ and Populus × euramericana CV. ‘74/76’. The cuttings were grown in a greenhouse (26 ± 5 °C) until 6–8 expanded leaves were produced and then were placed into a growth chamber (BIC-400, Boxun, Shanghai, China) for 1 week to acclimate to the conditions of 25/16 °C (day/night), a photoperiod of 16 h light (6 a.m.–10 p.m.) with a photosynthetic photon flux density (PPFD) of 125 ± 5 µmol m−2 s−1 at plant level, and 60% relative air humidity. To avoid SO2 absorbance by the soil substrate, pots were wrapped by the air tight plastic before starting the fumigation experiments.
Experimental design
Six poplar plants of each variety were placed in a Plexiglass enclosure (40 × 50 × 90 cm3) and exposed to SO2 using an experimental fumigation system referred to the design by Randewig et al. (2012). The enclosure was equipped with a fan (ACF-120, Golden Filed, Beijing, China), and leaf temperature was measured with a thermocouple (copper-constantan thermocouple, Type ‘T’) positioned on the lower leaf surface. The temperature and relative humidity in the enclosure were monitored with a combined humidity and temperature sensor (HMP155, Vaisala, Finland). Light in the controlled-environment chamber was provided by fluorescent lamps (12 V, 58 W, Philips Master TLD Leuchtstofflampen, Germany). The enclosure was flushed with 10 L min−1 of filtered ambient air provided by a gas cylinder. Defined SO2 concentrations were adjusted by adding a defined amount of concentrated SO2 (250 μL L−1, Yiyang CO., Beijing, China) to the airstream by means of a mass flow controller (21S-1–55-1–5-KMB05, ALICATA, USA). Mass flow meters (21–1-00–1-20-KMB05, ALICATA, USA) were used to control the flow rates into the enclosure. The airstream was connected to a SO2 analyzer (AP-G008, Anpaer, Shenzhen, China). Leaf temperature, enclosure temperature, and relative humidity were recorded in a PC-based data acquisition system (CR1000, Campbell Scientific, USA) with intervals of 24 min.
The plants in the Plexiglass enclosure were exposed to 0.7, 1.4, and 2.1 μL L−1 SO2 for 5 h. Under the highest concentration of SO2 (2.1 μL L−1 SO2), the susceptible variety of P. × euramericana cv. '74/76' exhibited visible symptoms of injury induced by SO2 (Fig. 1), while the resistant variety of P. × euramericana cv. 'Purui' did not have visible symptoms. The moderate concentration of 1.4 μL L−1 SO2 caused phenotypical symptoms of injury with small necrotic spots on the leaves of the susceptible variety. The lower concentration of SO2 did not induce visible symptoms regardless resistant or susceptible variety. Later on, we chose the moderate concentration of 1.4 μL L−1 SO2 to treat the plants, and compared their responses in the activity of enzymes, the content of metabolites, and the expression of enzymatic genes in pathways of sulfur metabolism. The leaf samples exposed to 1.4 μL L−1 SO2 were harvested and immediately frozen in liquid nitrogen and stored at – 80 °C for RNA extraction and biochemical analysis. The 5th and the 6th expanded leaf, counted from the apex, were pooled for biochemical analysis, and the 4th and the 5th leaf were pooled for RNA extraction. The treatment was repeated three times with a new set of plants. A parallel fumigation experiment with air served as the control.
RNA extraction, transcriptome sequencing, gene expression analysis, and functional annotation
Total RNA of each sample was extracted using a Quick RNA extraction kit (Bioteke Corporation, Beijing, China). RNA was purified and examined with a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The RIN (RNA integrity number) values (> 8.0) of these samples were assessed using an Agilent 2100 Bioanalyzer (Santa Clara, CA, USA). The construction of the libraries and the RNA-Seq were performed by the Biomarker Biotechnology Corporation (Beijing, China). The cDNA libraries were paired-end sequenced using an Illumina HiSeq™ 2000. The clean reads were mapped to the Populus genome database (Phytozome10, Populus trichocarpa v. 3.0) using TopHat2 (Kim et al. 2013). Transcript levels are presented as reads per kilobase of exon model per million mapped reads (RPKM) values (Florea et al. 2013). Differentially expressed genes (DEGs) were identified using the following criteria: Fold Change ≥ 2 and false discovery rate (FDR) ≤ 0.01, and DESeq was used to analyze the differential expression between sample groups (Anders and Huber, 2010). Pathway annotation of differentially expressed genes was based on the KEGG (Kyoto Encyclopedia of Genes and Genomes) databases (http://www.genome.jp/kegg/kegg2.html) (Gerlich and Neumann 2000).
Gene validation of expression analysis
The cDNA synthesis was obtained from total RNA using the SuperScript III First-Strand Synthesis System (Promega) according to the manufacturer’s instructions. Control reactions without a template were performed for each primer pair. RT-q-PCR was performed using a LightCycler 480 instrument (Roche Applied Science, Indianapolis, IN, USA). At least two independent biological replicates and four technical replicates of each biological replicate for each sample were analyzed by q-PCR to ensure reproducibility and reliability. Normalization was performed using three stably expressed reference genes (UBQ-L, αTub, and ACT2). The relative quantification value for each target gene was compared to those three reference genes. Annotation of differentially expressed genes on the pathway of sulfur, glutathione, cysteine, and methionine metabolism was focused and subjected to real-time quantitative PCR (q-PCR) with specific primers identified by Primer3 (Supplementary Table S1).
Quantification of enzyme activities
Total protein was extracted from frozen leaves using the Plant Total Protein Extraction Kit (Sigma) and detected at a wavelength of 595 nm using a microplate ELISA reader (SpectraMax 190, Molecular Devices, USA). For calibration, different amounts of bovine serum albumin (Sigma-Aldrich) were measured as a standard.
SiR activity was determined using the method as described by Randewig et al. (2012) with some modification. For SiR activity measurement, 10 μL total protein was diluted in 90 mL Tris acetate buffer (pH 7.8) to a final volume of 100 μL. The obtained solution is enzyme solution, and 20 μL enzyme solution was added to 80 μL reaction buffer (25 mM HEPES pH 7.8; 1 mM Na2SO3; 5 mM OAS-HCl, 10 mM DTT, 30 mM NaHCO3, 15 mM Na2S2O4, and 5 mM reduced methylviologen). The enzyme reaction was started by pipetting 20 μL of diluted enzyme solution into the reaction buffer and was stopped at 15 min by adding 50 μL of 20% TCA. After adding 100 μL glacial acetic acid and 200 μL of ninhydrine reagent (250 mg ninhydrine, 6 mL concentrated acetic acid, and 4 mL concentrated HCl), the light absorption of the reaction solution was measured at a wavelength of 560 nm using a microplate ELISA reader. For calibration, different amounts of cysteine were measured as a standard.
O-acetylserine(thiol) lyase (OAS-TL) and serine acetyltransferase (SAT) activity was measured using the method as described by Hartmann et al. (2000) with some modification. Briefly, homogenized frozen leaf material (0.1 g) was added to 3 mL pre-cool extraction buffer (30 mM Tris–HCl with 10 mM DTT) and then centrifuged for 20 min at 14,000 g at 4 °C. The enzyme assay contained 0.1 mL of supernatant and 0.1 mL reaction buffer (50 mM K2HPO4-KH2PO4 (pH = 7.5), 5 mM DTT, 10 mM O- acetylserine, 2 mM Na2S) for OAS-TL determination, or 0.1 mL reaction buffer (4 mM serine, 2 mM acetyl-CoA, 50 mM K2HPO4-KH2PO4 (pH = 7.5), 0.5 mM DTT, and 1 mM Na2S) for SAT determination. The mixture was incubated at 25 °C for 10 min (for OAS-TL assay): and for 30 min (for SAT assay). Then, 50 μL 20% TCA was added to the reaction solution which then was centrifuged for 20 min at 14,000 g at 4 °C. The supernatant was transferred to the test tube, to which 100 μL glacial acetic acid and 200 μL of ninhydrine reagent (250 mg ninhydrine, 6 mL concentrated acetic acid, 4 mL concentrated HCl) were added. The mixture was boiled for 10 min, and then, 550 μL 95% ethyl alcohol was added. After quick-cooling, the light absorption of the reaction solution was measured at a wavelength of 560 nm using a microplate ELISA reader. For calibration, different amounts of cysteine were measured as a standard.
For APR activity measurements, frozen leaf material (100 mg) was homogenized in extraction buffer [100 mM mono-/dipotassium phosphate buffer (pH 7.7) including 10 mM Na2SO3, 0.5 mM AMP, 10 mM DTT, 5 mM sodium EDTA, 10 mM L-Cys, 1% Triton X-100, and 2% polyvinylpyrrolidone (PVP40)] to a final volume of 3 mL, and then centrifuged for 10 min at 12,000 g at 4 °C. APR activity measurement followed the method by Trüper and Rogers (1971). The reaction mixture consisted of aliquots of 1 mL extract with 3 mL reaction buffer [50 mM Tris–HCl buffer (pH = 7.2) including 1.2 μmol/L AMP, 1.5 μmol/L K3Fe(CN)6, 12 μmol/L Na2SO3 and 24 μmol/L EDTA]. Light absorption was measured at a wavelength of 420 nm using a microplate ELISA reader. For calibration, different amounts of bovine serum albumin (Sigma-Aldrich) were measured as a standard.
SO activity was determined using the method by Randewig et al. (2014) with some modification. Frozen leaf material (1.8 g) was grinded in liquid nitrogen and mixed with extraction buffer (100 mM Tris acetate buffer pH 7.5, 10 mM KCl, 1 mM EGTA, 1 mM EDTA, 10 mL glycerin, freshly added: 2% v/v Triton X-100, 2% w/v PEG 1500, 2% v/v PMSF, 0.2% w/v ascorbic acid) to a final volume of 3 mL, and then centrifuged for 10 min at 12,000 g at 4 °C. Protein was re-dissolved in 0.6 mL modified resuspension buffer (50 mM HEPES pH 7.4, 1 mM EDTA, 6 mM DTT, 0.5 mM PMSF). Protein quantification was determined with the method of Bradford (1976), with Coomassie brilliant blue G-250 (mainly binds to basic or aromatic amino acid residues). Light absorption was measured at a wavelength of 595 nm using a microplate ELISA reader. For calibration, different amounts of bovine serum albumin (Sigma-Aldrich) were measured as a standard. For measuring SO activity, 200 μL of the protein extract was diluted in 200 μL Tris acetate buffer pH 7.25 to a final volume of 400 μL. Reaction was started by adding 100 μL of 0.5 mM sulfite. Quantification of sulfite reduction was measured using a solution containing formaldehyde and acid fuchsin for stopping the enzyme reaction and visualizing the color change by the formation of sulfate mediated by SO (Lang et al. 2007). The correlation between sulfite content (0–200 μM) and absorption values at 580 nm was determined for calculating SO activity (data for calibration curves not shown).
Metabolite determination
Quantification of thiols
The thiol content was determined by Agilent 1200 high-pressure liquid chromatography (HPLC) system (water 2695 HPLC, USA) (Ju et al. 2011). Homogenized frozen leaf material (200 mg) was extracted with extraction buffer (5 mM DTPA, 0.1% TFA, pH = 3.7) and then centrifuged for 10 min at 12,000 g at 4 °C. One mL supernatant was added to 2.6 mL HEPES buffer (5 mM DTPA, 200 mM HEPES, pH = 9) and 0.1 mL TCEP (20 mM with HEPES buffer) and incubated at 25 °C for 10 min in the dark, and then, 80 μL monobromobimane (50 mM with ACN) was added. The solution was derivatived at 25 °C for 30 min, and then, 400 mL MSA (1 mol·L−1) was added for stopping reaction. The derivative samples were filtered through 0.22 µm Nylon filter membrane (Millipore Corp., USA) and transferred to Agilent brown head empty bottle at 4 °C. Determination of thiol content was performed by fluorometric analysis (excitation at 380 nm, emission at 470 nm) with an HPLC Monitor and accounted by external standard method according to the apex apices area. The moving phase A was 0.1% TFA, and the moving phase B was 100% acetonitrile. The procedure was: 0–20 min, 8%-26% B; 20–22 min, 26%-100% B; 22–24 min, 100% B; 24–28 min, 100%-8% B; 28–30 min, 8% B.
Quantification of sulfate
Leaf SO42− content was quantified using anion exchange chromatograph (Dionex ICS-3000) (Brychkova et al. 2007). Homogenized oven-dried leaf material (0.1 g) was added to 1 mL deionized water containing 100 mg PVPP. The samples were shaken for 1 h at 4 °C, boiled for 15 min, and then centrifuged for 10 min at 15,000 g at 4 °C. The supernatant was centrifuged again for 5 min and diluted 20 times. Sulfate was quantified using a calibration curve of increasing sulfate concentration (50–800 μM).
Statistical analyses
Statistical analyses were performed using SAS 10.0 (SAS Institute Inc., Cary, NC, USA). Data were subjected with two-way ANOVA (with variety and treatment as factors) to detect differences between treatments (SO2 exposure and controls) and between different poplar varieties. Before ANOVA, data were ln-transformed to meet the assumptions of homogeneity of variance and normality when necessary.
Results
Under ambient air condition, the difference of enzyme activities, metabolites, and gene expression between the two varieties
Under ambient air conditions, there were no significant differences in SO (P = 0.052), APR (P = 0.537), and OASTL (P = 0.441) activities (Fig. 2), and CYS (P = 0.937) and GSH (P = 0.150) contents (Fig. 3) in leaves between P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76'. While P. × euramericana cv. 'Purui' had higher SiR activity (Fig. 2c, P = 0.009) than P. × euramericana cv. '74/76', the latter had a significantly higher SAT activity (Fig. 2d) (1.2-fold, P = 0.006) compared with P. × euramericana cv. 'Purui'. P. × euramericana cv. 'Purui' had roughly 1.6-fold (P = 0.017) higher sulfate contents (Fig. 3a) in leaves compared with P. × euramericana cv. '74/76'.
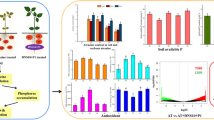
Activities of important sulfur metabolism enzymes in P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' in response to 1.4 µL L−1 SO2 for 5 h. Means and standard errors (n = 3) of enzyme activities are shown. Different capital letters indicate significant differences between the treatments within one poplar variety (P < 0.05). Different lower case letters indicate significant differences between the poplar varieties within one treatment (P < 0.05): a sulfite oxidase (SO), b adenosine 5′-phosphosulfate reductase (APR), c sulfite reductase (SIR), d serine acetyltransferase (SAT), and e O-acetylserine (thiol) lyase (OASTL). Purui-T is for SO2 treatment of P. × euramericana cv. 'Purui'; Purui-C for control of P. × euramericana cv. 'Purui'; 107-T for SO2 treatment of P. × euramericana cv. '74/76'; 107-C for control of P. × euramericana cv. '74/76'
Sulfur metabolites in controls and SO2-exposed (1.4 µL L−1 SO2 for 5 h) P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' poplars. Means and standard errors (n = 3) of sulfur metabolites are shown. The differences of sulfur metabolites were analyzed using two-way ANOVA (with variety and treatments as factors); a sulfate, b cysteine, and c glutathione. Different capital letters indicate significant differences between the treatments within one poplar variety (P < 0.05). Different lower case letters indicate significant differences between the poplar varieties within one treatments (P < 0.05). Purui-T is for SO2 treatment of P. × euramericana cv. 'Purui'; Purui-C for control of P. × euramericana cv. 'Purui'; 107-T for SO2 treatment of P. × euramericana cv. '74/76'; 107-C for control of P. × euramericana cv. '74/76'
For assimilation sulfate reduction, sulfate has to be activated by the ATP sulfurylase (ATPS) to form adenosine 5′-phosphosulfate (APS), which catalyzes the first step in this pathway. APS is converted into PAPS (3´-phosphoadenosine 5´-phosphosulfate) by 3'-phosphoadenosine 5'-phosphosulfate synthase (PAPSS), and the activated sulfate is partly converted into APS by PAPSS (Supplementary Fig. f). PAPSS in P. × euramericana cv. '74/76' was higher than that in P. × euramericana cv. 'Purui' (Fig. 4a). For glutathione metabolism, glutathione (GSH) partly converted into l-cysteinyl-glycine by gamma-glutamyltranspeptidase (E2.3.2.2) or glutathione hydrolase (E3.4.19.13) (ggt) (Supplementary Fig. g). The ggt in P. × euramericana cv. '74/76' was lower than that in P. × euramericana cv. 'Purui'. The low sulfate may be attributed to the relatively higher PAPSS activity that consumes the sulfate. On the other hand, higher SiR activity in P. × euramericana cv. 'Purui' did not result in more CYS and GSH contents, probably because the higher ggt may keep the organic sulfur substances in balance.
Schematic views of enzyme activities and S metabolites between P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' varieties in ambient air and SO2-exposed conditions. a P. × euramericana cv. 'Purui' vs P. × euramericana cv. '74/76' in ambient air condition, b P. × euramericana cv. 'Purui' vs P. × euramericana cv. '74/76' in SO2-exposed condition, c Control vs SO2 treatment of P. × euramericana cv. 'Purui', d Control vs SO2 treatment of P. × euramericana cv. '74/76'. Increasing metabolite levels and enzyme activities are marked with a thick red up arrow. A red word indicates that the enzyme is up-regulated by gene expression. A green word indicates that the enzyme is down-regulated by gene expression
After SO2 exposure, the difference of enzyme activities, metabolites, and gene expression between the two varieties
After SO2 exposure, SO, APR, and SiR enzyme activities of P. × euramericana cv. 'Purui' were higher (1.3-fold, P = 0.046; 1.3-fold, P = 0.003; 1.3-fold, P = 0.007) than those of P. × euramericana cv. '74/76' (Fig. 2). There were no significant differences in SAT (Fig. 2d P = 0.053) and OASTL (Fig. 2e P = 0.627) activities between the two varieties. P. × euramericana cv. 'Purui' had roughly 2.0-fold (P = 0.002) higher sulfate contents (Fig. 3a) and higher GSH contents (Fig. 3c) in leaves than P. × euramericana cv. '74/76'. There was no significant difference in CYS (Fig. 3b P = 0.357) content between the two varieties.
For sulfate metabolism, there was no significant difference in the process between P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' after exposure to SO2 (Fig. 4b). For cysteine and methionine metabolism, l-homocysteine converted into l- methionine by homocysteine S-methyltransferase (mmuM or BHMT2) or 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase (metE), l-methionine (Met) partly converted into S-Adenosyl- l-methionine (SAM) by S-adenosylmethionine synthetase (metK). SAM partly converted into S-adenosyl- l-homocysteine by DNA (cytosine-5)-methltransferase 1 (DNMT1 OR dcm). S-adenosyl- l-homocysteine converted into l- momcysteine by adenosylhomocysteinase (ahcY). On the other hand, 4-methylthion-2-oxobutanoate converted into l-methionine by tyrosine aminotransferase (TAT) (Supplementary Fig. h). After SO2 exposure, mmuM, metE, ahcY, and TAT were down-regulated in P. × euramericana cv. '74/76' compared with Purui poplar variety (Fig. 4b).
Effect of SO2 on enzyme activities, metabolites, and gene expression in P. × euramericana cv. 'Purui'
There were significant differences in the all five enzymes and two organic thiol compounds in leaves of P. × euramericana cv. 'Purui' between SO2 exposure treatment and the control (exposure to ambient air). SO, APR, SiR, SAT, and OASTL activities increased in response to SO2 by 27% (P = 0.045), 44% (P < 0.001), 28% (P = 0.007), 54% (P < 0.001), and 32% (P = 0.010), respectively (Fig. 2). P. × euramericana cv. 'Purui' after exposure to SO2 had a significantly higher CYS (1.4-fold, P = 0.019) (Fig. 3b) and GSH contents (2.1-fold, P = 0.008) (Fig. 3c) than that under ambient air condition. However, there was no significant difference in sulfate between SO2 exposure and the control (Fig. 3a P = 0.112).
For cysteine and methionine metabolism, sulfide and O-acetyl- l-serine together synthetize l-cysteine by l-3-cyanoalanine synthase (ATCYSC1) or cysteine synthase (cysK), which produces organic sulfocompounds from sulfide (Supplementary Fig. a, b). Under SO2 fumigation, l-3-cyanoalanine synthase (ATCYSC1) in P. × euramericana cv. 'Purui' was down-regulated compared with that in ambient air condition (Fig. 4c). Met was partly converted into SAM by S-adenosylmethionine synthetase (metK), SAM was partly converted into 1-aminocyclopropane-1-carboxylate (ACC) by 1-aminocyclopropane-1-carboxylate synthase (ACS) (Supplementary Fig. b). MetK and ACS were up-regulated in Purui poplar variety by SO2 fumigation (Fig. 4c). For glutathione metabolism, glutathione (GSH) together with RX partly converted into R-S-glutathione by glutathione S-transferase (GST) (Ye and Song 2005) (Supplementary Fig. c). GST was up-regulated in P. × euramericana cv. 'Purui' after SO2 fumigation compared with that in ambient air condition.
Effect of SO2 on enzyme activities, metabolites, and gene expression in P. × euramericana cv. ‘74/76’
SO, APR, SiR, SAT, and OASTL activities increased in response to SO2 by 37% (P = 0.086), 10% (P = 0.152), 37% (P = 0.009), 16% (P = 0.011), and 18% (P = 0.073), respectively (Fig. 2). SiR (P = 0.009) and SAT (P = 0.011) activities in P. × euramericana cv. ‘74/76’ after SO2 fumigation were higher than those under ambient air condition, while there were no significant differences in SO (P = 0.086), APR (P = 0.152), and OASTL (P = 0.073) activities in P. × euramericana cv. ‘74/76’ over the two different treatments (Fig. 2). Sulfate (P = 0.979) levels, CYS (P = 0.076), and GSH (P = 0.496) contents of P. × euramericana cv. '74/76' were not changed by the SO2 treatment (Fig. 3).
For sulfate metabolism, PAPSS in P. × euramericana cv. '74/76' was down-regulated by the SO2 treatment (Fig. 4d). For cysteine and methionine metabolism, sulfide and O-acetyl-l-serine together synthetize l-cysteine by cysteine synthase (cysK), which produces organic sulfocompounds from sulfide (Supplementary Fig. e). Under SO2 fumigation, cysteine synthase (cysK) in P. × euramericana cv. '74/76' was down-regulated compared with that in ambient air condition. Met partly converted into S-adenosyl- l-methionine (SAM) by S-adenosylmethionine synthetase (metK) and partly converted into methanethiol by methionine-gamma-lyase (E4.4.1.11). SAM partly converted into 1-aminocyclopropane-1-carboxylate (ACC) by 1-aminocyclopropane-1-carboxylate synthase (ACS) and partly converted into S-adenosyl-l-homocysteine by DNA (cytosine-5)-methltransferase 1 (DNMT1 or dcm) (Supplementary Fig. e). Methionine-gamma-lyase (E4.4.1.11) was down-regulated in P. × euramericana cv. '74/76' by SO2 fumigation. MetK, DNMT1 and ACS were up-regulated in P. × euramericana cv. '74/76' by SO2 fumigation (Fig. 4d).
Discussion
The different detoxification mechanisms in coping with SO2 between the two varieties
Most poplar species are ranked as highly SO2 sensitive up to intermediately tolerant (Kozlowski 1980), but clonal variations in pollution resistance are also well known for poplar species (Karnosky 1976, 1977). Populus × euramericana cv. ‘purui’ has strong survival ability in the high SO2 pollution environment with some characteristics of SO2 tolerance (Xu et al. 2011); however, the metabolite mechanisms of detoxification of sulfur dioxide toxic gases is not clear. Previous experiments with model plant Arabidopsis thaliana have identified the detoxification mechanisms of sulfur-containing gases, namely oxidative detoxification (sulfite is oxidized into sulfate) or reductive detoxification (sulfite is reduced to sulfide) (Van der Kooij et al. 1997; Lang et al. 2007; Brychkova et al. 2012, 2013; Hamisch et al. 2012; Randewig et al. 2012). The capacity of sulfite-to-sulfate oxidation is determined by measuring SO activity. To survive under high atmospheric SO2 concentration, plants need the presence of effective SO, and it is one of the main mechanisms to remove excess sulfite in Arabidopsis (Lang et al. 2007; Randewig et al. 2012). In the present study, the SO activity in P. × euramericana cv. 'Purui' increased after SO2 exposure, but not in P. × euramericana cv. '74/76' (Fig. 2a). On the other hand, SiR-defective mutants revealed higher sensitivity to SO2, whereas SiR-overexpressing plants showed higher resistance (Brychkova et al. 2013; Yarmolinsky et al. 2013). In the present study, the accumulation of Cys and GSH resulted from a strong increase of the SO and SiR activities in P. × euramericana cv. 'Purui' after SO2 exposure. However, the only SiR activity was increased in P. × euramericana cv. '74/76' and the Cys and GSH remained unchanged after SO2 exposure (Fig. 2c). From the current results, it is evident that both sulfite oxidation and sulfite reduction as well as the assimilation contribute to SO2 detoxification in Purui poplar variety, which is consistent to the finding with Populus × canescens (Randewig et al. 2014). The detoxification mechanism of P. × euramericana cv. '74/76' is only by reductive detoxification with SiR to copy with SO2. By contrast, P. × euramericana cv. 'Purui' increases not only SO but also SiR activity, contributing to sulfite detoxification via the sulfite network, which realizes sufficient sulfite reduction for Cys synthesis on one hand and prevents cells from toxic sulfite levels on the other hand. This mechanism is consistent to these previous studies (Brychkova et al. 2013; Yarmolinsky et al. 2013).
APR is one of the main control enzymes in the sulfate assimilation pathway (Kopriva 2006; Khan et al. 2010). The activity of APR is controlled by the negative feedback of reduced sulfur compounds (Tsakraklides et al. 2002). It has been previously reported that exposure of plants to SO2 can lead to such a down regulation (Randewig et al. 2014; Brunold et al. 1983; Tschanz et al. 1986). The experiment with Populus × canescens is conducted with different SO2 concentrations (0.65, 0.8, 1.0, and 1.2 µL L−1) exposed for approximately 3 days. The APR activity is down-regulated and thiol contents increased further with increasing SO2 concentrations (up to 1.0 µL L−1 SO2). However, the accumulation of Cys and GSH tends to decline after exposure to 1.2 µL L−1 SO2. This indicates that sulfur assimilation may be disturbed during exposure to 1.2 µL L−1 SO2 that is the critical level for P. × canescens (Randewig et al. 2014). In this study, the APR activity of P. × euramericana cv. '74/76' remained unchanged, while that of P. × euramericana cv. 'Purui' was increase after exposure to 1.4 µL L−1 SO2 (Fig. 2b). The increased activity of the APR enzyme can lead to the production of more sulfite, SO32−. However, when the activity of APR enzyme is decreased, the content of sulfate will increase. Although SO42− is less toxic than SO32−, it is associated with stomatal closure in SO2 polluted environments (Robinson et al. 1998). The sulfate level in leaves of P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' varieties remained unchanged after SO2 fumigation (Fig. 3a). While the accumulation of Cys and GSH in leaves of P. × euramericana cv. 'Purui' tended to increase after exposure to 1.4 µL L−1 SO2, the Cys and GSH levels in leaves of P. × euramericana cv. '74/76' remained unchanged after SO2 fumigation (Fig. 3b, c). This might indicate that sulfur assimilation became obviously disturbed during exposure to 1.4 µL L−1 SO2 for P. × euramericana cv. '74/76'. Cysteine and methionine are sulfur-containing amino acids. CYS is the first organic compound containing reduced sulfur synthesized by the plant (Takahashi et al. 2011). In plants, cysteine is converted by transferring hydrogen sulfide to serine (via acetylserine). The surplus sulfide might have been used as a macronutrient after assimilation into organic sulfur compounds, such as Cys, for growth and development in Purui poplar variety. On the other hand, the APR activity enhanced and hence improved reduction capacities, which might contribute to maintain the sulfate stability of P. × euramericana cv. 'Purui'. In addition, GSH is an important antioxidant responsible for maintenance of the antioxidative machinery of the cells under stress (Nagalakshmi and Prasad 2001).
There is a regulatory association of SAT and SiR activities to promote the flux through the sulfate reduction to sulfide pathway (Berkowitz et al. 2002; Riemenschneider et al. 2005; Scheerer et al. 2010). Promotion of this flux appears appropriate to prevent enhanced sulfite levels during SO2 fumigation in cooperation with enhanced availability of OASTL for cysteine synthesis. From the present experiment, OASTL activity significantly increased in P. × euramericana cv. 'Purui', but not in P. × euramericana cv. '74/76', although both P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' increased SAT and SiR activities in response to SO2 fumigation. An increased cysteine after SO2 exposure in P. × euramericana cv. 'Purui', not in P. × euramericana cv. '74/76', is also evident.
The difference in transcriptional regulation of enzymes related to sulfur metabolism between the two varieties coping with SO2
After SO2 exposure, P. × euramericana cv. 'Purui' up-regulated mmuM, metE, ahcY, and TAT expression compared with P. × euramericana cv. '74/76' variety (Fig. 4b). At the same time, P. × euramericana cv. 'Purui' poplar also up-regulated SO and SiR activity, which can convert toxic sulfite into nontoxic sulfate and organic sulfur substances, such as GSH and CYS. The sulfate and GSH accumulation in P. × euramericana cv. 'Purui' is evident for that. On the other hand, the up-regulated expressions of some enzymes genes may promote the cysteine and methionine metabolism and cysteine conversion, which may be a reason why CYS did not accumulated.
For assimilation sulfate reduction, sulfate has to be activated to form APS by the ATP sulfurylase (ATPS) and APS is converted into PAPS (3´-phosphoadenosine 5´-phosphosulfate) by 3'-phosphoadenosine 5'-phosphosulfate synthase (PAPSS). On the other hand, the activated sulfate is partly converted into APS by PAPSS. In the current results, transcripts encoding PAPSS in P. × euramericana cv. '74/76' was down-regulated in response to SO2 exposure, which could affect the generation of APS and PAPS, and the consumption of sulfite. Thus, the decrease of PAPSS is not conducive for P. × euramericana cv. '74/76' to detoxify sulfite.
Glutathione S-transferases (GST) were up-regulated in P. × euramericana cv. 'Purui' after SO2 fumigation, indicating that a growing number of toxic intermediate metabolites are converted into innocuous substances through combining GSH (Gill and Tuteja 2010; Giraud et al. 2012). GSTs are ubiquitous proteins in plants that play important roles in stress tolerance and detoxification metabolism (Lan et al. 2009; Chan and Lam 2014). In addition, under SO2 fumigation, L-3-cyanoalanine synthase (ATCYSC1) in P. × euramericana cv. 'Purui' and cysteine synthase (cysK) in P. × euramericana cv. '74/76' were down-regulated. It is estimated that SO2 will affect the cysteine content of plants in the future.
It is also controversial over SO transcriptional regulation in response to SO2 stress. Plants with over-expressed SO gene increase sulfite oxidation capacity and hence adapt to high concentrations of SO2 (Brychkova et al. 2007). With Arabidopsis SO knockout lines, Hamisch et al. (2012) have found that the two splice variants of the SO gene are up-regulated under mild SO2 stress, which provides evidence for the co-regulation between SO and APR at the mRNA level. However, Arabidopsis transcripts encoding SO were not significantly changed in the SO2-fumigated plants (Zhao and Yi 2014). In this study, we found that transcripts encoding SO also were not significantly changed in both P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' after SO2 fumigation, although SO activity in P. × euramericana cv. 'Purui' was increased after SO2 exposure. Transcripts encoding APR and sulfate transporter (SULTR) were down-regulated in the chloroplast upon SO2 exposure (Hamisch et al. 2012). However, we found that transcripts encoding SO, APR, SULTR, and SiR were not significantly changed in P. × euramericana cv. 'Purui' and P. × euramericana cv. '74/76' after SO2 fumigation.
Conclusion
SO2 enters leaf cells through leaf stomata and combines with water to produce sulfite SO32−, which is considered to be toxic to plants. P. × euramericana cv. 'Purui' can survive in the environment with high concentration of sulfur dioxide, and it has two ways of detoxification: (1) oxidation detoxification, oxidizing sulfite (SO32−) to sulfate (SO42−) by sulfite oxidase (SO); (2) reductive detoxification, SO32− is reduced to S2− by sulfite reductase (SiR). By contrast, P. × euramericana cv. '74/76'' is sensitive to sulfur dioxide, and it detoxifies sulfite only through reduction. In this study, it was found that after SO2 fumigation, the activity of APR enzyme increased and the content of SO42− remained unchanged in P. × euramericana cv. 'Purui'. Thus, we speculate that P. × euramericana cv. 'Purui' could reduce and consume SO42− produced by the oxidation pathway of SO with increasing the activity of APR enzyme, as to maintain the stability of SO42− content in cells. In addition, the increased activity of sulfite reductase (SiR) will reduce SO32− to S2−, which is the donor of all sulfur-containing amino acids. This study also found increased OASTL and SAT activity, and increased levels of cysteine (CYS) and glutathione (GSH) in P. × euramericana cv. 'Purui' in response to SO2 fumigation. Cysteine is a precursor of methionine (MET), and the expression of the gene encoding s-adenosine methionine synthetase (metK) is up-regulated. We speculate that more methionine (MET) will be converted to s-adenosine-l-methionine (SAM). The up-regulated expression of genes encoding 1-amino-cyclopropane-1-carboxylate synthetase (ACS) suggests that more s-adenosine-l-methionine (SAM) will be converted into 1-amino-cyclopropane-1-carboxylate (ACC). This process makes more surplus cysteine (CYS) to be utilized. Under the concentration of 1.4 µL L−1 SO2, the sulfur metabolism of P. × euramericana cv. 'Purui' is not much disturbed. The absorbed SO2 can be detoxified through the metabolic pathway, and the metabolites generated can be used for the growth of P. × euramericana cv. 'Purui' in the polluted environment. This may be the reason that P. × euramericana cv. 'Purui' can survive in the environment polluted by high concentration of SO2. For P. × euramericana cv. '74/76' with weak resistance to sulfur dioxide, it only detoxifies sulfite by increasing the activity of sulfite reductase (SiR), and down-regulates the expression of gene encoding 3'-phosphoadenosine 5'-phosphosulfate synthase (PAPSS) under SO2 stress, which will affect the generation of 3´-phosphoadenosine 5´-phosphosulfate (PAPS), and the consumption of sulfite.
After SO2 exposure, P. × euramericana cv. 'Purui' up-regulated mmuM, metE, ahcY, and TAT expression compared with P. × euramericana cv. '74/76' variety. The up-regulated expressions of those enzymes genes may promote the cysteine and methionine metabolism and GSH accumulation. In addition, glutathione S-transferases (GST) were up-regulated in P. × euramericana cv. 'Purui' after SO2 fumigation, suggesting that a growing number of toxic intermediate metabolites are converted into innocuous substances through combining GSH. However, there is no strong correlation between the expression of some enzymatic genes in pathways of sulfur metabolism and the response to SO2 stress, probably because the post-transcriptional processing of the genes may play a regulatory role.
Abbreviations
- SO2 :
-
Sulfur dioxide
- SO:
-
Sulfite oxidase
- SiR:
-
Sulfite reductase
- SAT:
-
Serine acetyltransferase
- OAS:
-
O-acetylserine
- OASTL:
-
O-acetylserine (thiol) lyase
- APR:
-
Adenosine 5′-phosphosulfate reductase
- APS:
-
Adenosine 5′-phosphosulfate
- Cys:
-
Cysteine
- γ-EC:
-
γ-Glutamylcysteine
- GSH:
-
Glutathione
- PAPSS:
-
3'-Phosphoadenosine 5'-phosphosulfate synthase
- ATCYSC1:
-
L-3-cyanoalanine synthase
- cysK:
-
Cysteine synthase
- mmuM:
-
Homocysteine S-methyltransferase
- metE:
-
5-Methyltetrahydropteroyltriglutamate-homocysteine methyltransferase
- E4.4.1.11:
-
Methionine-gamma-lyase
- ACS:
-
1-Aminocyclopropane-1-carboxylate synthase
- DNMT1:
-
DNA (cytosine-5)-methltransferase 1
- ahcY:
-
Sdenosylhomocysteinase
- TAT:
-
Tyrosine aminotransferase
- ggt:
-
Gamma-glutamyltranspeptidase (E2.3.2.2) or glutathione hydrolase (E3.4.19.13)
- GST:
-
Glutathione S-transferase
- Met:
-
L-methionine
- SAM:
-
S-adenosyl-L-methionine
- ACC:
-
1-Aminocyclopropane-1-carboxylate
References
Alscher R (1984) Effects of SO2 on light-modulated enzyme reactions. In: Koziol MJ, Whatley FR (eds) Gaseous air pollutants and plant metabolism. Butterworths, London, pp 181–200
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106
Asada K, Kiso K (1975) Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur J Biochem 33:253–257
Berkowitz O, Wirtz M, Wolf A, Kuhlmann J, Hell R (2002) Use of biomolecular interaction analysis to elucidate the regulatory mechanism of the cysteine synthase complex from Arabidopsis thaliana. J Biol Chem 277:30629–30634
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brunold C, Landolt W, Lavanchy P (1983) SO2 and assimilatory sulfate reduction in beech leaves. Physiol Plant 59:313–318
Brychkova G, Xia Z, Yang G, Yesbergenova Z, Zhang Z, Davydov O, Fluhr R, Sagi M (2007) Sulfite oxidase protects plants against sulfur dioxide toxicity. Plant J 50:696–709
Brychkova G, Yarmolinsky D, Fluhr R, Sagi M (2012) The determination of sulphate level and its oxidation in plant leaves. Plant Sci 190:123–130
Brychkova G, Grishkevich V, Fluhr R, Sagi M (2013) An essential role for tomato sulfite oxidase and enzymes of the sulfite network in maintaining leaf sulfite homeostasis. Plant Physiol 161:148–164
Buchner P, Takahashi H, Hawkesford MJ (2004) Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J Exp Bot 55:1765–1773
Chan C, Lam HM (2014) A putative lambda class glutathione s-transferase enhances plant survival under salinity stress. Plant Cell Physiol 55:570–579
Eilers T, Schwarz G, Brinkmann H, Witt C, Richter T, Nieder J, Koch B, Hille R, Hänsch R, Mendel RR (2001) Identification and biochemical characterization of Arabidopsis thaliana sulfite oxidase. a new player in plant sulfur metabolism. J Biol Chem 276:46989–46994
Filner P, Rennenberg H, Sekiya J, Bressan RA, Wilson LG, LeCureux L, Shimei T (1984) Biosynthesis and emission of hydrogen sulfide by higher plants. In: Koziol MJ, Whatley FR (eds) Gaseous Air Pollutants and Plant Metabolism. Butterworth, London, UK, pp 291–312
Florea L, Song L, Salzberg SL (2013) Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues [v2; ref status: indexed, http://f1000r.es/2dl]. F1000Research 2:188.
Gerlich M, Neumann S (2000) Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giraud E, Ivanova A, Gordon CS, Whelan J, Considine MJ (2012) Sulfur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant Cell Environ 35:405–417
Grulke NE, Neufeld HS, Davison AW, Roberts M, Chappelka AH (2007) Stomatal behavior of ozone-sensitive and –insensitive coneflowers (Rudbeckia laciniata var. Digitata ) in Great Smoky Mountains National Park. New Phytol 173:100–109
Hamisch D, Randewig D, Schliesky S, Bräutigam A, Weber APM, Geffers R, Herschbach C, Rennenberg H, Mendel RR, Hänsch R (2012) Impact of SO2 on Arabidopsis thaliana transcriptome in wildtype and sulfite oxidase knockout plants analyzed by RNA deep sequencing. New Phytol 196:1074–1085
Hartmann T, Mult S, Suter M, Rennenberg H, Herschbach C (2000) Leaf age-dependent differences in sulphur assimilation and allocation in poplar (Poplus tremula × P. alba) leaves. J Exp Bot 51:1077–1088
Heber U, Hüve K (1998) Action of SO2 on plants and metabolic detoxification of SO2. Int Rev Cytol 177:255–286
Ju X-H, Tang S-R, Jia Y et al (2011) Determination and characterization of cysteine, glutathione and phytochelatins (PC2-6) in Lolium perenne L. exposed to Cd stress under ambient and elevated carbon dioxide using HPLC with fluorescence detection. J Chromatogr B 879:1717–1724
Karnosky DF (1976) Threshold levels for foliar injury to Populus tremuloides by sulfur dioxide and ozone. Can J for Res 6:166–169
Karnosky DF (1977) Evidence for genetic control of response to sulfur dioxide and ozone in Populus tremuloides. Can J Res 7:437–440
Khan MS, Haas FH, Samami AA et al (2010) Sulphite reductase defines a newly discovered bottleneck for assimilatory sulphate reduction and is essential for growth and development in Arabidopsis thaliana. Plant Cell 22:1216–1231
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36
Kondo N (2002) Uptake, Metabolism, and Detoxification of Sulfur Dioxide. Omasa K, Saji H, Youssefian S and Kondo N. Air Pollution and Plant Biotechnology. Springer (India) Private Limited Publishing, Tokyo, pp 179–199
Kopriva S (2006) Regulation of sulfate assimilation in Arabidopsis and beyond. Ann Bot Lond 97:479–495
Kozlowski TT (1980) Responses of shade trees to pollution. J Arboric 6:29–41
Lan T, Yang Z-L, Yang X et al (2009) Extensive Functional Diversification of the Populus Glutathione S-Transferase Supergene Family. Plant Cell 21:3749–3766
Lang C, Popko J, Wirtz M, Hell R, Herschbach C, Kreuzwieser J, Rennenberg H, Mendel RR, Hänsch R (2007) Sulfite oxidase as key enzyme for protecting plants against sulfur dioxide. Plant Cell Environ 30:447–455
Lendzian KJ (1984) Permeability of plant cuticles to gaseous air pollutants. In: Kozoil MJ, Whatley FR (eds) Gaseous Air Pollutants and Plant Metabolism. Butterworths, pp 77–81
Li C, McLinden C, Fioletov V, Krotkov N, Carn S, Joiner J, Streets D, He H, Ren XR, Li ZQ, Dickerson RR (2017) India is overtaking china as the world’s largest emitter of anthropogenic sulfur dioxide. Sci Rep 7:14304. https://doi.org/10.1038/s41598-017-14639-8
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Pfanz H, Dietz K-J, Weinerth I, Oppmann B (1990) Detoxification of sulfur dioxide by apoplastic peroxidases. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I (eds) Sulfur nutrition and sulfur assimilation in higher plants. SPB Academic Publishing, The Hague, the Netherlands, pp 229–233
Randewig D, Hamisch D, Herschbach C, Eiblmeier M, Gehl C, Jurgeleit J, Skerra J, Mendel RR, Rennenberg H, Hänsch R (2012) Sulfite oxidase controls sulfur metabolism under SO2 exposure in Arabidopsis thaliana. Plant, Cell Environ 35:100–115
Randewig D, Hamisch D, Eiblmeier K, Boedecker C, Kreuzwieser J, Mendel RR, obert Hnsch R, Herschbach C, Rennenberg H, (2014) Oxidation and reduction of sulfite contribute to susceptibility and detoxification of SO2 in Populus ×canescens leaves. Trees 28:399–411
Rennenberg H, Herschbach C (2014) A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite flux analyses. J Exp Bot 65:5711–5724
Riemenschneider A, Riedel K, Hoefgen R, Papenbrock J, Hesse H (2005) Impact of reduced O-acetylserine(thiol)lyase isoform contents on potato plant metabolism. Plant Physiol 137:892–900
Robinson MF, Heath J, Mandfield TA (1998) Disturbances in stomatal behavior caused by air pollutants. J Exp Bot 49:461–469
Scheerer U, Hänsch R, Mendel RR, Kopriva S, Rennenberg H, Herschbach C (2010) Sulfur flux through the sulfate assimilation pathway is differently controlled by adenosine 5′-phosphosulfate reductase under stress and in transgenic poplar plants overexpressing gamma-ECS, SO, or APR. J Exp Bot 61:609–622
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulphur assimilation in photosynthetic organism: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Tamm CO, Cowling EB (1977) Acid precipitation and forest vegetation. Water Air Soil Pollut 7:503–511
Trüper HG, Rogers LA (1971) Purification and properties of adenylyl sulfate reductase from the phototrophic sulfur bacterium, Thiocapsa roseopersicina. J Bacteriol 108:1112–1121
Tsakraklides G, Martin M, Chalam R, Tarczynski MC, Schmidt A, Leustek T (2002) Sulfate reduction is increased in transgenic Arabidopsis thaliana expressing 5-adenylylsulfate reductase from Pseudomonas aeruginosa. Plant J 32:879–889
Tschanz A, Landoit W, Bleuler P, Brunold C (1986) Effect of SO2, on the activity of adenosine 50-phosphosulfate sulfotransferase from spruce trees (Picea abies) in fumigation chambers and under field conditions. Physiol Plant 67:235–241
Van der Kooij TAW, De Kok LJ, Haneklaus S, Schnug E (1997) Uptake and metabolism of sulfur dioxide by Arabidopsis thaliana. New Phytol 135:101–107
Xu J, Bai KD, Wan XC, Cheng GH, Zhang CY, Zhang ZX (2011) Responses of poplar (Populus × euramericana cv.“74 /76”) SO2-resistant clone to SO2 fumigation and the variation in antioxidant systems. Scientia Silvae Sinicae 47(2):66–71
Yarmolinsky D, Brychkova G, Fluhr R, Sagi M (2013) Sulfite reductase protects plants against sulfite toxicity. Plant Physiol 161:725–743
Ye Z, Song H (2005) Glutathione s-transferase polymorphisms (GSTM1, GSTP1 and GSTT1) and the risk of acute leukaemia: A systematic review and meta-analysis. Eur J Cancer 41:980–989
Zhao J, Yi H (2014) Genome-wide transcriptome analysis of Arabidopsis response to sulfur dioxide fumigation. Mol Genet Genomics 289:989–999
Funding
The research was supported by Fundamental Research Funds of Research Institute of Forest New Technology (CAFYBB2018SZ019) and (CAFINT2014K09), Chinese Academy of Forestry, Beijing, China.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: JF and XW. Performed the experiments: JF, LW, WL, ZCh, and JZ. Analyzed the data: JF, LW, and XW. Wrote the paper: JF and XW.
Corresponding author
Additional information
Communicated by V.P. Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, J., Wang, L., Liu, W. et al. Differences in detoxification mechanism and gene expression changes of sulfur metabolism in coping with the air pollutant SO2 between the resistant and ordinary poplar variety. Acta Physiol Plant 44, 124 (2022). https://doi.org/10.1007/s11738-022-03442-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03442-2