Abstract
This study was conducted to determine if common buckwheat (Fagopyrum esculentum Moench) residues affect a phenolics composition in soil, and are effective for limiting emergence, growth and metabolic changes in barnyard grass (Echinochloa crus-galli (L.) P. Beauv.), wild oat (Avena fatua L.), yellow foxtail (Setaria pumila Schult.), silky windgrass (Apera spica-venti L.), catchweed bedstraw (Galium aparine L.), scentless mayweed (Matricaria inodora L.), and tiny vetch (Vicia hirsuta L.). In the study, the residues of 14-day-old buckwheat plants were used (cv. Hruszowska). After removal of the above-ground parts, the buckwheat root residues (BRR) remained in the soil for an additional 7 days before the weeds were sown. For comparison, under the same cultivation conditions, the effect of entire buckwheat plant residues (BPR) in soil was assessed. BPR and BRR in the soil caused a decrease in the emergence of all weed species except the tiny vetch. The growth of barnyard grass, wild oat, yellow foxtail, catchweed bedstraw, and scentless mayweed was inhibited by BRR, but not BPR. BRR had a particularly strong inhibitory effect on the growth of scentless mayweed (SM) and catchweed bedstraw (CB). Thirty-day-old SM and CB control plants were about 4 and 3.5 times higher, respectively, than plants growing in the presence of BRR. Furthermore, chlorophyll and carotenoid contents in the barnyard grass and catchweed bedstraw were more prominently reduced by BRR than BPR. Stressful conditions caused by buckwheat residues in the soil increased the level of phenolic compounds in the tissues of weeds examined. Soil with buckwheat residues contained a low level of phenolic compounds, which may indicate their slow release from the residue or rapid utilisation by microorganisms. These phenolic compounds probably cannot be directly responsible for allelopathic inhibition of weed emergence and growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can control weeds by allelochemicals secreted by roots and/or through decomposition of plant residues (Batish et al. 2002; Xuan et al. 2005). This complex phenomenon is affected by the condition of the soil, age of plants and climatic conditions (Kobayashi 2004). Studies on allelopathy mainly involve the effect of extracts obtained from various parts of the plant, and leaves which appear to be the most important source of chemicals involved in phytotoxicity (Tanveer et al. 2010). As our earlier results have shown, aqueous extracts obtained from the above-ground parts of buckwheat are stronger inhibitors of seed germination and seedling growth of wheat and lettuce compared to extracts obtained from roots (Mioduszewska et al., 2013). Interestingly, an aqueous extract from the root of fennel (Foeniculum vulgare) showed a higher allelopathic effect on goosefoot (Chenopodium album L.) than a shoot extract, although it had low antioxidant activity and trace phenolic content (Aryakia et al. 2015).
Another type of allelopathic research is based on incorporation into soil of fresh organic matter which releases phytotoxins and/or induces increased microbial activity which affects seeds germination and growth of seedlings (Mohler et al. 2012, 2018). Allelochemicals can be released from crop residues in soil or they can be produced by microorganisms that use these residues as a substrate (Kruidhof et al. 2011). The following are responsible for the suppression by crop residues of seedling emergence and growth: released allelochemicals, reduction of nitrates by soil microbes and an increase in pathogen population and composition (Kumar et al. 2008, 2009). Soils containing residues of various plants exhibited reduced seedling emergence of Abutilon theophrasti, Chenopodium album, Amaranthus powellii, Setaria faberi and Echinochloa crus-galli by an average of 30% (Mohler et al. 2012). The authors concluded that the depression in emergence rate was caused by a biological agent. This conclusion is based on the comparison of emergence rates in sterilised and unsterilised soil. Time between residue incorporation into the soil and emergence of receptor plant is an additional factor for explaining the variation in inhibition of emergence among plants (Kruidhof et al. 2011).
Common buckwheat, often used as a cover crop, has a short growing season and is able to compete with many weed species (Björkman and Shail 2013). Buckwheat residues can also affect the presence and population of soil microorganisms. Potato tubers grown in soil containing buckwheat residues had significantly lower Verticillium wilt ratings than potato from control plots (Wiggins and Kinkel 2005). Buckwheat can inhibit weed growth by root exudates containing allelochemicals. Kalinova et al. (2007) showed that 4-hydroxyacetophenone, vanillic and gallic acids from buckwheat roots inhibit the growth of Amaranthus retroflexus, Achillea millefolium and Sinapis alba. Common buckwheat has been cultivated to control quackgrass (Elymus repens L.) in Poland for hundreds of years (Tsuzuki and Dong 2003; Golisz et al. 2007a, b; Falquet et al. 2015). Tominaga and Uezu (1995) showed that extracts from the soil in which buckwheat had grown inhibited the root growth of barnyard grass and purslane (Portulaca oleracea) but not affected the growth of Galinsoga ciliata.
Buckwheat residues incorporated into soil might inhibit weed emergence and growth (Iqbal et al. 2003; Xuan and Tsuzuki 2004). Kumar et al. (2008) found that buckwheat residues in soil reduced the emergence and biomass of some weed species, such as redroot pigweed, shepherd’s purse, and corn chamomile, but not inhibited growth of barnyard grass. Buckwheat residues in soil also strongly hampered the growth of field pennycress (Thlaspi arvense), gallant soldier (Galinsoga parviflora), Canada thistle (Cirsium arvense) and narrowleaf plantain (Plantago lanceolata) (Kalinova 2004). In studies by Gfeller et al. (2018), growth suppression of redroot pigweed, goosefoot and barnyard grass was found when weeds were co-cultivated with buckwheat.
Phenolic compounds released by plant residues present in soil are considered to be one of the main factors causing allelopathic effects for plants growing there (Blum et al. 1999; Batish et al. 2002, 2007). In the soil, phenolics might be degraded and they can polymerise and condense with humic substances, adsorb to clay minerals or remain in a dissolved form (Blum 2004; Cecchi et al. 2004; Rabinovich et al. 2004; Li et al. 2013). In soils, phenolics are mainly degraded by fungi and bacteria. Various strains of microbes effectively and rapidly decomposed p-coumaric, ferulic, p-hydroxybenzoic and trans-cinnamic acids (Zhang et al. 2010; Xie and Dai 2015). A variety of solvents have been applied to extract phenolic compounds in soils, e.g.: water, acetone, methanol and citrate solution. Citrate solution is effective for extracting phenolic acids bound to soil particles (Blum 1997).
Numerous allelopathic compounds, including flavonoids, phenolic acids, fatty acids, and alkaloids, were identified in common buckwheat tissues (Iqbal et al. 2002; Golisz et al. 2007a, b; Kalinova et al. 2007; Kalinova and Vrchotova 2009). However, there is no clear evidence which buckwheat compounds are directly responsible for its allelopathic properties. It is likely that a whole complex of compounds from this species, as well as microorganisms present in the soil are involved in this proces (Wirth and Gfeller 2016; Gfeller et al. 2018).
The following phenolic acids have been determined in the hypocotyl and cotyledons of buckwheat seedlings: o-, m-, and p-coumaric acids (2-, 3-, and 4-hydroxycinnamic), sinapic acid (4-hydroxy-3,5-dimethoxycinnamic), caffeic acid (3,4-dihydroxycinnamic), and two isomers of ferulic acid (4-hydroxy-3-methoxycinnamic and 3-hydroxy-4-methoxycinnamic) (Horbowicz et al. 2015; Wiczkowski et al. 2016). Moreover, hydroxybenzoic, protocatechuic, gallic and syringic benzoic acid derivatives have also been found in both the buckwheat organs. Major phenolic acids in buckwheat cotyledons are o-coumaric acid and sinapic acid, while caffeic and chlorogenic acid in the hypocotyl.
Most studies on buckwheat allelopathic features focus on the impact of extracts from the crop tissues or the residues of entire plants in the soil. An influence of 1% aqueous extract obtained from 14-day-old common buckwheat plants on wild oat (Avena fatua L.), yellow foxtail (Setaria glauca L.), barnyard grass (Echinochloa crus-galli L.), common windgrass (Apera spica-venti (L.), catchweed bedstraw (Galium aparine L.), scentless mayweed (Matricaria inodora L), gallant soldier (Galinsoga parviflora Cav.) and tiny vetch (Vicia hirsuta L.) has been examined recently (Szwed et al. 2019a). The obtained results showed that the buckwheat extract exerted less influence on the growth of shoots than roots of the test species. The buckwheat extract added to the nutrient medium resulted in an inhibition of root growth of all the species except tiny vetch. In the case of weed shoot, growth inhibition by the buckwheat extract occurred only in wild oat. Moreover, the buckwheat extract activated an antioxidant response and increased the content of most phenolic acids and flavonoids in the test weed species. We have also demonstrated that there are differences in an impact on weeds of entire buckwheat plant residues and buckwheat root residues in the soil (Szwed et al. 2019b). These results showed an inhibition of barnyard grass and cleavers biomass accumulation caused by buckwheat root residues in the soil.
The studies presented here are a continuation of our previous work (Szwed et al. 2019b) and were carried out to assess the effect of buckwheat residues on the emergence, growth and basic metabolic reactions of weed seedlings and to check whether phenolic compounds released to soil from the buckwheat residues are related to allelopathic features of this species.
Materials and methods
Plant material and growth conditions
European monocotyledonous and dicotyledonous weed species that display strong competitive interactions were examined: barnyard grass (Echinochloa crus-galli (L.) P. Beauv.), wild oat (Avena fatua L.), yellow foxtail (Setaria pumila Schult.) and silky windgrass (Apera spica-venti L.), and dicotyledonous weeds included: catchweed bedstraw (Galium aparine L.), scentless mayweed (scentless chamomile) (Matricaria inodora L.), and tiny vetch (hairy vetch) (Vicia hirsuta L.).
Weed seeds were collected from a natural population in arable fields in the vicinity of Radzyń Podlaski (51°48′ N, 22°38′ E, eastern Poland). In the case of yellow foxtail and catchweed bedstraw, seed dormancy was broken by two-week wet stratification. Vetch seeds were scarified as described by Kimura and Islam (2012). The seeds were then sterilised with 70% ethanol and sodium hypochlorite and stored at room temperature in dark and dry conditions.
The study was conducted in controlled conditions in a pot experiment with temperature 24 ± 2 °C during the day (16 h) and 18 ± 2 °C during the night (8 h). PAR (100–120 µmol m−2 s−1) was provided by high-pressure sodium lamps (400 W, Plantaster, Osram, Germany). Seeds of common buckwheat, cv. Hruszowska (ca. 300; 10 g per one container), were sown containers (80 × 15 × 15 cm; length × with × depth) filled with 6 kg (18 dm3) of commercial garden soil (HIT-TORF, pH = 6.48; 6.9 g N kg−1; 0.65 g P kg−1, Poland). The containers were watered in the morning as needed. After 14 days of buckwheat plant growth, the above-ground parts of buckwheat plants at 4 containers were removed. In another set of 4 containers the above-ground parts were cut with a knife and thoroughly mixed with roots and the soil. In the third set of containers buckwheat was not cultivated. An initial root biomass (BRR) in the soil of one container was 193 ± 32 g, and whole buckwheat plants (BPR) was 521 ± 44 g, on average. After dividing the containers into parts using glass plates, a different species of weeds were sown in each of them. The total number of sown weed seeds was 100–120. The same species were sown in each of the four containers.
Assay of emergence rate and growth of weeds
Each combination (control, BPR, and BRR) consisted of four containers in which weed seeds were sown (at the depth of 2–3 cm). Emergence rate was estimated 7 days after sowing. The height of above-ground parts of weeds was determined after 30 days from sowing (DAS). The mean height of plants from each container was considered one replicate.
Analysis of phenolic compounds in soil
The profile and content of phenolic acids and flavonoids in soil after 7 days of decomposition of buckwheat residues were determined according to the method described previously (Wiczkowski et al. 2016; Szwed et al. 2019b). Extracts were obtained from 50- to 200-g soil samples by shaking with water, citrate, acetone and methanol for 1 day, and then concentrated to dryness in a freeze dryer. Pulverised samples were analysed by HPLC–MS/MS for the concentration of various forms of phenolic acids and flavonoids (free, esters, and glycosides).
Other analytical details were described previously (Szwed et al. 2019b). The following phenolic compounds were analysed in soil extracts: ferulic acid, p-coumaric acid, chlorogenic acid, caffeic acid, sinapic acid, vanillic and iso-vanillic acid, protocatechuic acid, syringic acid, luteolin, apigenin, kaempferol, and quercetin were. Each phenolic compound was quantified based on the HPLC–MS/MS peak area at the appropriate multiple reaction monitoring (MRM) according to the corresponding linear calibration curves (0.01–0.5 μg mL−1).
Analysis of metabolites in weeds
Analyses of the influence of BRR and BPR in soil on some biochemical indicators of weed species were carried out for barnyard grass, as a representative of monocotyledonous plants, and catchweed bedstraw, as a dicotyledonous plant. The measurements were taken in above-ground parts of weeds at the end of the experiment (30 DAS) which were freeze-dried and pulverised in a laboratory mill. Total soluble protein measurements were made using the Bradford method (1976). Samples of fresh plant tissues were homogenised with potassium phosphate buffer (pH 7.0) containing 4% (w/v) polyvinylpyrrolidone. The homogenate was centrifuged for 0.5 h at 12,000 g at 4 °C. Bradford reagent was added to the obtained supernatant. The samples were incubated at room temperature and absorbance was measured at 595 nm. The protein content was read from the standard curve prepared for known concentrations of bovine albumin.
The rate of lipid peroxidation process was analysed on the basis of measurements of the malondialdehyde (MDA) in freeze-dried plant tissues. After homogenisation with 80% ethanol content of MDA was measured as a result of the reaction with thiobarbituric acid (TBA). Samples were heated at 95 °C for 0.5 h, and after cooling were centrifuged (0.5 h, 3000g) and absorbance at 440, 532 and 600 nm was measured, and appropriate formulae were used to calculate MDA content (Hodges et al. 1999).
Chlorophyll a and b, and total carotenoids were analysed in 80% acetone extracts of freeze-dried and pulverised plant tissues. Samples were extracted for 24 h at room temperature in darkness with occasional shaking, and after filtration, quantified with a spectrophotometer (645, 883 and 470 nm) using formulas published by Lichtenthaler and Wellburn (1985)
Total phenolics were determined spectrophotometrically using Folin–Ciocalteau reagent (Singleton et al. 1999). Phenolics from freeze-dried tissue were extracted with 80% ethanol. The extracts were diluted with water and Folin–Ciocalteau reagent was added. Reagent mixtures were allowed to react at ambient temperature in darkness for 2 h, and the absorbance of supernatants was measured at 725 nm. Chlorogenic acid was used for a standard curve preparation.
Anthocyanin content was measured as described by Mancinelli (1984). Freeze-dried samples were extracted with 1% HCl in methanol for 24 h at room temperature in darkness with occasional shaking. The extracts were filtered and their absorbance was measured at 530 and 657 nm. For calculation of anthocyanins content the formula A530–0.25A657 was used to compensate for the absorption of chlorophylls degradation products. Anthocyanin content was calculated using a molecular extinction coefficient of cyanidin-3-glucoside (29,600).
The method described by Ordonez et al. (2006) was used for the analysis of the total flavonoid content. Freeze-dried samples were homogenised with 80% ethanol, the extract was filtered, and after dilution with water, 5% sodium nitrite was added. After 5 min. of incubation, 10% aluminium chloride was added. The samples were incubated for 5 min. and 1-M NaOH was added. The absorbance was measured at 510 nm. The total flavonoid content was calculated on the basis of a standard curve made for rutin.
A spectrophotometer UV-1800 UV/Vis, Rayleigh, was used to take the measurements of proteins, photosynthetic pigments, total phenolics, and MDA.
Statistical analyses
Analysis of variance (one-way ANOVA) and Tukey’s post hoc test were used to check the significance of differences. Calculations were performed using Statistica 12 (StatSoft, Poland).
Results
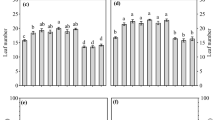
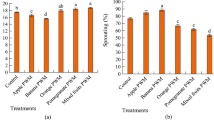
The presence of BPR and BRR in the soil caused a decline in an emergence of all the weed species, except tiny vetch (Fig. 1). BPR caused the highest reduction of emergence (more than twice compared to control) in the case of windgrass, yellow foxtail, catchweed bedstraw and scentless mayweed. A particularly large reduction in emergence rate was caused by BRR in the case of catchweed bedstraw and scentless mayweed, the suppression being nine- and fivefold higher, respectively, compared to control. The emergence decline due to BRR was significantly higher than that caused by BPR. The average emergence rates for dicotyledonous and monocotyledonous weeds under the influence of BPR and BRR were 31.5 and 29.4%, respectively, and were significantly lower than for control plants (52.2%).
Effect of entire buckwheat plant (BPR) and buckwheat root (BRR) in the soil on emergence of weed species. 1, barnyard grass (Echinochloa crus-galli L.); 2, wind grass (Apera spica-venti L.); 3, wild oat (Avena fatua L.), 4, yellow foxtail (Setaria pumila Schult.), 5, catchweed bedstraw (Galium aparine L.); 6, scentless mayweed (Matricaria inodora L.) 7, tiny vetch (Vicia hirsuta L.). Results (mean SD) marked with the same letter are not significantly different at P < 0.05
BRR inhibited the seedling growth of barnyard grass, wild oat, yellow foxtail, catchweed bedstraw, and scentless mayweed, and the inhibition was significantly higher than in the presence of BPR (Fig. 2). BPR had no effect on the growth of these weeds, but it stimulated the growth of wind grass seedlings. Similar to the influence on emergence, BRR greatly inhibited catchweed bedstraw and scentless mayweed growth (by 71 and 85%, respectively). For barnyard grass and yellow foxtail, growth decline reached 41 and 28%, respectively.
Effect of entire buckwheat plant (BPR) and buckwheat root (BRR) in the soil on growth of weed species (expressed as a height of above-ground parts). 1, barnyard grass (Echinochloa crus-galli L.); 2, wind grass (Apera spica-venti L.); 3, wild oat (Avena fatua L.), 4, yellow foxtail (Setaria pumila Schult.), 5, catchweed bedstraw (Galium aparine L.); 6, scentless mayweed (Matricaria inodora L.) 7, tiny vetch (Vicia hirsuta L.). Results (mean SD) marked with the same letter are not significantly different at P < 0.05
BRR evidently decreased soluble proteins in barnyard grass leaves and epicotyls, being twice as low as in the tissues of control plants (Table 1). Also, BPR reduced proteins in epicotyls of barnyard grass, but increased their level in catchweed bedstraw leaves and stems. BRR significantly increased the accumulation of phenolic compounds (total phenols, total flavonoids and anthocyanins) in both test weed species (Table 1). The impact of BPR on levels of these compounds was lower and not always statistically significant. In general, the level of malondialdehyde (MDA) increased under the influence of BPR and BRR, although the significance of this increase was demonstrated only for barnyard grass epicotyls in plants grown in the presence of BRR (Table 1).
Chlorophyll a and b contents in the tissues of barnyard grass and catchweed bedstraw were reduced by BPR and BRR (Table 2). Levels of the chlorophylls in barnyard grass tissues were more than twice as low as in the presence of BRR in soil compared with control plants, while BPR reduced them by only about 40%.
Also in catchweed bedstraw leaves, BRR significantly reduced chlorophyll contents, being about 3.5 times as low as in the leaves of control plants (Table 2). The influence of BPR and BRR on the level of total carotenoids in weed tissues was low (Table 2). However, BRR was found to be a stronger inhibitor of the accumulation of these pigments than BPR.
After a seven-day decomposition of buckwheat residues, only a few phenolic compounds present in buckwheat tissues occurred in the soil extracts (Tables 3 and 4). Water extracts contain ortho-, meta- and para-coumaric acids, and low levels of apigenin. The share of free forms of these compounds was low, and they appeared mainly as esters. The level of phenolic acids and apigenin in the sodium citrate extract was much higher than in the aqueous extract. In this case, also ester forms were present at the highest concentrations. When acetone and methanol were used for soil extraction, p-coumaric, ferulic and caffeic acids were found. Among these acids, only p-coumaric acid was found at relatively high levels, caffeic acid being found at trace amounts. As with the aqueous extract, esters were the primary form of these acids.
Apart from the acids listed in Tables 3 and 4, the following acids determined by HPLC–MS method were absent in soil extracts: sinapic, trans-cinnamic, chlorogenic, gallic, protocatechuic, vanillic and syringic acid. Quercetin, luteolin and kaempferol derivatives were not found, either. It is worth noting that the content of phenolic compounds in the control soil in many cases was higher or equal to that in the soil with root residues (BRR) or whole buckwheat plants (BPR). And in several cases, phenolics content in soil with BRR was even higher than in soil containing BPR.
Discussion
In studies on allelopathic weed control, measurements of seed germination and seedling growth in in vitro culture are mainly used (Yang et al. 2002; Kalinova et al. 2007; Golisz et al. 2007a, b; Oyerinde et al. 2009; Kaur et al. 2012). In soil conditions, however, the remains of entire buckwheat plants are used to assess the allelopathic effect on weeds (Iqbal et al. 2003; Xuan and Tsuzuki 2004; Kumar et al. 2008, 2009). Results of these studies showed that common buckwheat was effective for suppressing the growth of quackgrass (Elymus repens L.) (Golisz et al. 2007a, b), amaranth (Amaranthus powellii), shepherd's-purse (Capsella bursa-pastoris), and corn chamomile (Anthemis arvensis) (Kumar et al. 2008, 2009). The residues of buckwheat in soil also curbed the growth of field pennycress (Thlaspi arvense L.), gallant soldier (Galinsoga parviflora), Canada thistle (Cirsium arvense) and narrowleaf plantain (Plantago lanceolata) (Kalinova 2004).
In our previous study, the biomass of 30-day-old barnyard grass (Echinochloa crus-galli) and catchweed bedstraw (Galium aparine) plants grown in bare soil was approximately 5- and 3.5-fold higher, respectively, than in plants grown in soil with buckwheat root residues (BRR) (Szwed et al. 2019b). The biomass of wind grass and tiny vetch plants was not affected by BRR but the antioxidant response was increased by raising peroxidase activity and content of phenolic compounds. This indicates that the weeds have adapted to stress conditions. It was demonstrated in the present study that BRR and BPR inhibited emergence of all weed species, except tiny vetch. Both types of residues had a particularly marked inhibitory effect on windgrass, catchweed bedstraw and scentless mayweed emergence. The growth inhibition of seedlings in the majority of the evaluated species was evident in the presence of BRR and not BPR. BRR significantly inhibited growth of 30-day-old seedlings of barnyard grass, wild oat, yellow foxtail, catchweed bedstraw, and scentless mayweed. These results partly confirm previous observations in which water extracts from the soil from buckwheat cultivation inhibited the elongation of barnyard grass roots under in vitro conditions (Tominaga and Uezu 1995; Kalinova and Vrchotova 2009). Furthermore, in recently described experiments, Gfeller et al. (2018) have found that barnyard grass height was reduced by 77% when it was grown together with buckwheat. Aryakia et al. (2015) also showed that water extracts from fennel roots (Foeniculum vulgare) had a higher allelopathic effect on goosefoot (Chenopodium album L.) than extracts from above-ground parts.
The reason why buckwheat root residues more effectively inhibit the growth of weeds than residues of entire buckwheat plants is not clear. It is assumed that the mechanism by which allelochemicals directly suppress weeds may be accompanied by indirect effects related to the control of fungal pathogens and competition for soil nutrients (Kumar et al. 2009). When the buckwheat residues are deposited in the soil, nitrogen can become immobilised, which can lead to a shortage of this element and thus additional growth restriction (Kumar et al. 2008, 2009). The time of incorporation of plants into the soil is also important for weed control to be effective (Kruidhof et al. 2011). It is commonly believed that allelopathic effects are usually not related to a single substance but a mixture of allelochemicals (Cheng and Cheng 2015). Therefore, it is possible that the decaying roots of buckwheat produce an active set of allelochemicals in soil, and the decomposition of entire plants can disturb production of these compounds directly or indirectly through microorganisms existing there, as was suggested by Kumar et al. (2009).
During decomposition of plant residues, phytotoxic compounds such as phenolics can be released into the soil. Buckwheat tissues rich in phenolic compounds were the subject of studies evaluating their allelopathic properties (Tominaga and Uezu 1995; Xuan and Tsuzuki 2004; Golisz et al. 2007a, b; Kalinova et al. 2007; Kalinova and Vrchotova 2009; Szwed et al. 2019a). However, current results showed that the content of these compounds in the soil was low and below 6 µg g−1, i.e., below 10–7 M. These results indicate that the direct allelopathic effect of phenolic compounds derived from decomposing buckwheat tissues is small or insignificant. Moreover, the share of free forms of these compounds was negligible. Their content in the control soil was generally equal to or even higher than in soil containing root residue (BRR) or whole buckwheat plants (BPR).
Most of the phenolic compounds present in buckwheat tissues were not found in soil extracts. No sinapic, trans-cinnamic, chlorogenic, gallic, protocatechuic, vanillic and syringic acid. were found in the soil. Quercetin, luteolin and kaempferol derivatives were not found, either. Furthermore, it is particularly surprising that the soil lacks trans-cinnamic acid which occurs at high concentrations in buckwheat tissues (Wiczkowski et al. 2016). This may indicate that they are probably rapidly chemically/biochemically transformed or used by soil micro-organisms. It may indicate that the direct effect of buckwheat phenolic compounds on inhibition of germination and growth of the test weed species is insignificant. Our results partly confirm the previously published data on the impact of buckwheat on redroot pigweed (Amaranthus retroflexus) (Wirth and Gfeller 2016). These authors suggest that buckwheat inhibiting the growth of redroot pigweed is not due to the presence of allelopathic compounds. The reason for this may be that buckwheat allelochemicals are rapidly degraded or their concentration in the soil solution is too low. An example of indirect influence of allelopathic compounds in soil is the fact that ferulic acid, p-hydroxybenzoic acid and hydroxamic acid may curb the population of soil microorganisms responsible for the nitrification process (Ma 2005). Another reason for this might be the fact that a kind of symbiosis or balance between roots and soil microorganisms is established during cultivation of buckwheat. On the basis of the root exudates, microorganisms can produce and release allelopathic compounds into the soil, thus reducing the emergence and growth of other plants. The introduction of above-ground parts of buckwheat (BPR) rich in various phenolic compounds into the soil probably disturbs this symbiosis and/or the microorganism population. As a result, the microorganisms may reduce the production of compounds that may have allelopathic properties. This hypothesis (or rather speculation) requires confirmation by extensive microbiological and chemical examination of the soil.
Another way that can cause changes in the composition and content of phenolic acids in soil is associated with lignin synthesis. Lignin, the end product of phenylpropanoid metabolism in plants, plays an important role in their resistance to stress conditions (Boerjan et al. 2003). It is known that lignins can be acylated by various phenolic acids, including derivatives of benzoic and cinnamic acids (Ralph 2010; Zhang et al. 2019).
The effect of phenolic compounds on a decline in chlorophyll and carotenoids contents and photosynthesis is known (Baziramakenga et al. 1994; Yang et al. 2002; Oyerinde et al. 2009). Reduction in the photosynthetic pigments may be associated with the inhibition of their biosynthesis and/or its degradation as a result of the allelopathic stress (Yang et al. 2002). Peng et al. (2004) have pointed out that many phenolic allelochemicals affect the photosynthesis and plant growth by destroying chlorophyll. The results obtained in this study indicate that the presence of buckwheat residues reduced the content of photosynthetic pigments: chlorophylls and carotenoids. In the tissues of barnyard grass and catchweed bedstraw, a high decrease in the accumulation of these pigments was particularly evident in the case of BRR.
The inhibition of growth and the decline in photosynthetic pigments was accompanied by a decrease in soluble proteins, which indicates that the presence of buckwheat residues interferes with the basic metabolic processes of weeds. The results confirm previous findings where allelochemicals from other plants caused a decrease in protein content (Padhy et al. 2000; Gulzar et al. 2014). Baziramakenga et al. (1997) suggest that this decrease is due to the interruption of the inclusion of certain amino acids in protein chains.
According to the available data, the accumulation of reactive oxygen species (ROS) in plant cells is affected by numerous allelopathic compounds present in the environment (Pergo and Ishii-Iwamoto 2011; Kaur et al. 2012). ROS decompose the polyunsaturated lipids, forming malonic dialdehyde (MDA) which is a biomarker of the magnitude of oxidative stress (Davey et al. 2000).
In response to the stress caused by buckwheat residues in the soil, higher levels of phenolic compounds, especially flavonoids, were accumulated in the tissues of barnyard grass and catchweed bedstraw. This was particularly evident under the influence of BRR. BRR also increased MDA levels in the leaves of barnyard grass, but not in catchweed bedstraw. Therefore, the results obtained should be treated with caution.
Conclusions
In a pot study, entire buckwheat plant residues (BPR) and buckwheat root residues (BRR) inhibited the emergence and growth of most test weed species. Growth of barnyard grass, wild oat, yellow foxtail, catchweed bedstraw, and scentless mayweed was inhibited by BRR, but not BPR. The height of 30-day-old control seedlings of scentless mayweed and catchweed bedstraw was approximately fourfold and 3.5-fold higher, respectively, than plants grown in the presence of BRR. Moreover, BRR had a stronger inhibitory effect on chlorophyll and carotenoid accumulation in tissues of examined weed species than BPR. BPR and especially BRR increased the level of phenolic compounds, especially flavonoids and anthocyanins in weed tissues. Soil with buckwheat residues contained a low level of phenolic compounds, which may indicate their slow release from these residues and/or rapid utilisation by microorganisms. A low concentration of phenolic compounds may not be directly responsible for allelopathic inhibition of emergence and growth of weeds.
Author contribution statement
MS, JM and HD carried out plant experiments and basic analyses; WW carried out LC–MS–MS analyses of phenolic compounds; MH contributed to data analysis and preparing of manuscript. All the authors have read and approved the revised manuscript.
References
Aryakia E, Naghavi MR, Farahmand Z, Shahzadeh Fazeli AAH (2015) Evaluating allelopathic effects of some plant species in tissue culture media as an accurate method for selection of tolerant plant and screening of bioherbicides. J Agric Sci Technol 17:1011–1023
Batish DR, Singh HP, Pandher JK, Aroroa V, Kohli RK (2002) Phytotoxic effect of Parthenium hysterophorus residues on the selected soil properties and growth of chickpea and radish. Weed Biol Manag 2:73–78
Batish DR, Lavanya K, Singh HP, Kohli PK (2007) Phenolic allelochemicals released by Chenopodium murale affect growth, nodulation and macromolecule content in ckickpea and pea. Plant Growth Regul 51:119–128
Baziramakenga R, Simard RR, Leroux GD (1994) Effects of benzoic and cinnamic acids on growth, mineral composition, and chlorophyll content of soybean. J Chem Ecol 20:282–2833. https://doi.org/10.1007/BF02098391
Baziramakenga R, Leroux GD, Simard RR, Nadeau P (1997) Allelopathic effects of phenolic acids on nucleic acid and protein levels in soybean seedlings. Can J Bot 75:445–450. https://doi.org/10.1139/b97-047
Björkman T, Shail JW (2013) Using a buckwheat cover crop for maximum weed suppression after early vegetables. HortTechno 23:575–580. https://doi.org/10.21273/HORTTECH.23.5.575
Blum U (1997) Benefits of citrate over EDTA for extracting phenolic acids from soils and plant debris. J Chem Ecol 23:347–362. https://doi.org/10.1023/B:JOEC.0000006364.17425.7
Blum U (2004) Fate of phenolic allelochemicals in soils—the role of soil and rhizosphere microorganisms. In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Allelopathy: chemistry and mode of action of allelochemicals. CRC Press, Boca Raton, pp 57–76
Blum U, Shafer SR, Lehman ME (1999) Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: concepts vs. an experimental model. Crit Rev Plant Sci 18:673–693. https://doi.org/10.1080/07352689991309441
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546. https://doi.org/10.1146/annurev.arplant.54.031902.134938
Bradford MM (1976) A dye binding assay for protein. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cecchi AM, Koskinen WC, Cheng HH, Haider K (2004) Sorption-desorption of phenolic acids as affected by soil properties. Biol Fert Soils 39(4):235–242. https://doi.org/10.1007/s00374-003-0710-6
Cheng F, Cheng Z (2015) Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci 6:1020. https://doi.org/10.3389/fpls.2015.01020
Davey MW, Stals E, Panis B, Keulemans J, Swennen RL (2000) High-throughput determination of malondialdehyde in plant tissues. Anal Biochem 347:201–207. https://doi.org/10.1016/j.ab.2005.09.041
Falquet BA, Gfeller A, Pourcelot M, Tschuy F, Wirth J (2015) Weed suppression by common buckwheat: a review. Environ Contr Biol 53:1–6. https://doi.org/10.2525/ecb.53.1
Gfeller A, Glauser G, Etter C, Signarbieux C, Wirth J (2018) Fagopyrum esculentum alters its root exudation after Amaranthus retroflexus recognition and suppresses weed growth. Front Plant Sci 9:50. https://doi.org/10.3389/fpls.2018.00050
Golisz A, Gawronska H, Gawronski SW (2007a) Influence of buckwheat allelochemicals on crops and weeds. Allelopathy J 19:337–349
Golisz A, Lata B, Gawronski SW, Fujii Y (2007b) Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol Manag 7:164–171. https://doi.org/10.1111/j.1445-6664.2007.00252.x
Gulzar A, Siddiqui MB, Bi S (2014) Assessment of allelopathic potential of Cassia sophera L. on seedling growth and physiological basis of weed plants. Afr J Biotech 13:1037–1046. https://doi.org/10.5897/AJB2013.1351
Hodges DM, DeLong JM, Forney ChF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. https://doi.org/10.1007/s004250050524
Horbowicz M, Wiczkowski W, Szawara-Nowak D, Sawicki T, Kosson R, Sytykiewicz H (2015) The level of flavonoids and amines in de-etiolated and methyl jasmonate treated seedlings of common buckwheat. Phytochem Lett 13:15–19. https://doi.org/10.1016/j.phytol.2015.05.011
Iqbal Z, Hiradate S, Noda A, Isojima SI, Fujii Y (2002) Allelopathy of buckwheat: assessment of allelopathic potential of extract of aerial parts of buckwheat and identification of fagomine and other related alkaloids as allelochemicals. Weed Biol Manag 2(2):110–115. https://doi.org/10.1046/j.1445-6664.2002.00055.x
Iqbal Z, Hiradate S, Noda A, Isojima SI, Fujii Y (2003) Allelopathic activity of buckwheat: isolation and characterization of phenolics. Weed Sci 51:657–662. https://doi.org/10.1614/0043-1745(2003)051[0657:AAOBIA]2.0.CO;2
Kalinova J (2004) Influence of common buckwheat on growth of other plant species. Proceedings of the 9th International Symposium on Buckwheat, Prague, pp. 529–531
Kalinova J, Vrchotova N (2009) Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J Agric Food Chem 57:2719–2725. https://doi.org/10.1021/jf803633f
Kalinova J, Vrchotova N, Triska J (2007) Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench.). J Agric Food Chem 55:6453–6459. https://doi.org/10.1021/jf070795u
Kaur S, Singh HP, Batish DR, Kohli RK (2012) Artemisia scoparia essential oil inhibited root growth involves reactive oxygen species (ROS)-mediated disruption of oxidative metabolism: in vivo ROS detection and alterations in antioxidant enzymes. Biochem System Ecol 44:390–399. https://doi.org/10.1016/j.bse.2012.06.015
Kimura E, Islam MA (2012) Seed scarification methods and their use in forage legumes. Res J Seed Sci 5:38–50. https://doi.org/10.3923/rjss.2012.38.50
Kobayashi K (2004) Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol Manag 4:1–7. https://doi.org/10.1111/j.1445-6664.2003.00112.x
Kruidhof HM, Gallandt ER, Haramoto ER, Bastiaans L (2011) Selective weed suppression by cover crop residues: effects of seed mass and timing of species sensitivity. Weed Res 51:177–186. https://doi.org/10.1111/j.1365-3180.2010.00825.x
Kumar V, Brainard DC, Bellinder RR (2008) Suppression of powell amaranth (Amaranthus powellii), shepherd's-purse (Capsella bursa-pastoris), and corn chamomile (Anthemis arvensis) by buckwheat residues: role of nitrogen and fungal pathogens. Weed Sci 56:271–280. https://doi.org/10.1614/WS-07-106.1
Kumar V, Brainard DC, Bellinder RR (2009) Suppression of powell amaranth (Amaranthus powellii) by buckwheat residues: role of allelopathy. Weed Sci 57:66–73. https://doi.org/10.1614/WS-08-028.1
Li LL, Li TL, Zhang GC, Zhang EP, Zang J, Wu ZC (2013) Degradation patterns of phenolic acids in soil. Allelopathy J 31:181–188
Lichtenthaler HK, Wellburn AR (1985) Determination of total carotenoids and chlorophylls A and B on leaf in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Ma YQ (2005) Allelopathic studies of common wheat (Triticum aestivum L.). Weed Biol Manag 5:93–104. https://doi.org/10.1111/j.1445-6664.2005.00164.x
Mancinelli AL (1984) Photoregulation of anthocyanin synthesis. VIII. Effects of light pretreatments. Plant Physiol 75:447–453. https://doi.org/10.1104/pp.75.2.447
Mioduszewska H, Klocek J, Horbowicz M, Wolska K (2013) Effect of water extracts from tissues of common buckwheat on seed germination and seedlings growth of winter wheat and lettuce. Acta Sci Pol Agric 12:45–54
Mohler CL, Dykeman C, Nelson EB, Ditommaso A (2012) Reduction in weed seedling emergence by pathogens following the incorporation of green crop residue. Weed Res 52:467–477. https://doi.org/10.1111/j.1365-3180.2012.00940
Mohler CL, Taylor A, DiTommaso A, Hahn R, Bellinder R (2018) Effects of incorporated rye and hairy vetch cover crop residue on the persistence of weed seeds in the soil. Weed Sci 66:379–385. https://doi.org/10.1017/wsc.2017.80
Ordonez AAL, Gomez JD, Vattuone MA, Isla MI (2006) Antioxidant activities of Sechium edule (Jacq.) swart extracts. Food Chem 97:452–458. https://doi.org/10.1016/j.foodchem.2005.05.024
Oyerinde RO, Otusanya OO, Akpor OB (2009) Allelopathic effect of Tithonia diversifolia on the germination, growth and chlorophyll contents of maize (Zea mays L.). Sci Res Ess 4:1553–1558
Padhy B, Patinaik PK, Tripathy AK (2000) Allelopathic potential of Eucalyptus leaf litter leachates on germination and seedling growth of fingermillet. Allelopathy J 7:69–78
Peng SL, Wen J, Guo QF (2004) Mechanism and active variety of allelochemicals. Acta Bot Sin 46:757–766
Pergo ÉM, Ishii-Iwamoto EL (2011) Changes in energy metabolism and antioxidant defense systems during seed germination of the weed species Ipomoea triloba L. and the responses to allelochemicals. J Chem Ecol 37:500–513. https://doi.org/10.1007/s10886-011-9945-0
Rabinovich ML, Bolobova AV, Vasilchenko LG (2004) Fungal decomposition of natural aromatic structures and xenobiotics: a review. Appl Biochem Microbiol 40:1–17. https://doi.org/10.1023/B:ABIM.0000010343.73266.08
Ralph J (2010) Hydroxycinnamates in lignification. Phytochem Rev 9:65–83. https://doi.org/10.1007/s11101-009-9141-9
Szwed M, Mitrus J, Horbowicz M (2019a) Allelopathic effect of buckwheat extract on seedlings of selected weed species. Agric Sci LXXIV(4):83–93
Szwed M, Wiczkowski W, Szawara-Nowak D, Obendorf RL, Horbowicz M (2019b) Allelopathic influence of common buckwheat root residues on selected weed species. Acta Physiol Plant 41:92. https://doi.org/10.1007/s11738-019-2885-y
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Tanveer A, Rehman A, Javaid MM, Abbas RN, Sibtain M, Ahmad AU, Ibin-I-Zamir MS, Chaudhary KM, Aziz A (2010) Allelopathic potential of Euphorbia helioscopia L. against wheat (Triticum aestivum L.), chickpea (Cicer arietinum L.) and lentil (Lens culinaris Medic.). Turk J Agric Forest 34:75–81. https://doi.org/10.3906/tar-0903-53
Tominaga T, Uezu T (1995) Weed suppression by buckwheat. In: T. Matano, A. Ujihasa (Eds.) Current advances in buckwheat research, vol. 2, Proceedings of the 6th international symposium of buckwheat, Nagano, Japan, pp. 693–697
Tsuzuki E, Dong YJ (2003) Buckwheat allelopathy: use in weed management. Allelopathy J 12:1–11
Wiczkowski W, Szawara-Nowak D, Sawicki T, Mitrus J, Kasprzykowski Z, Horbowicz M (2016) Profile of phenolic acids and antioxidant capacity in organs of common buckwheat sprout. Acta Aliment Hung 45(2):250–257. https://doi.org/10.1556/066.2016.45.2.12
Wiggins BE, Kinkel LL (2005) Green manures and crop sequences influence potato diseases and pathogen inhibitory activity of indigenous Streptomycetes. Phytopathology 95:178–185. https://doi.org/10.1094/PHYTO-95-0178
Wirth J, Gfeller A (2016) Is growing buckwheat allelopathic? Julius-Kühn-Archiv (Julius Kühn Institut, Bundesforschungsinstitut für Kulturpflanzen, Germany) 452:431–438. https://doi.org/10.5073/jka.2016.452.057
Xie XG, Dai CC (2015) Biodegradation of a model allelochemical cinnamic acid by a novel endophytic fungus Phomopsis liquidambari. Int Biodeter Biodegr 104:498–507. https://doi.org/10.1016/j.ibiod.2015.08.004
Xuan TD, Tsuzuki E (2004) Allelopathic plants: buckwheat. Allelopathy J 13:137–148
Xuan TD, Tawata S, Khanh TD, Chung MI (2005) Decomposition of allelopathic plants in soil. J Agric Crop Sci 191:162–171. https://doi.org/10.1111/j.1439-037X.2005.00170.x
Yang C, Lee C, Chou C (2002) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: I. Inhibition of supply-orientation. Bot Bull-Acad Sin Taipei 43:299–304
Zhang ZY, Pan LP, Li HH (2010) Isolation, identification and characterization of soil microbes which degrade phenolic allelochemicals. J Appl Microbiol 108:1839–1849. https://doi.org/10.1111/j.1365-2672.2009.04589
Zhang Y, Legland D, El Hage F, Devaux MF, Guillon F, Reymond M, Méchin V (2019) Changes in cell walls lignification, feruloylation and p-coumaroylation throughout maize internode development. PLoS ONE 14(7):e0219923. https://doi.org/10.1371/journal.pone.0219923
Acknowledgements
This work was supported in part by Ministry of Science and Higher Education (Poland) as the statutory activities of the Siedlce University of Natural Sciences and Humanities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Gniazdowska-Piekarska.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szwed, M., Mitrus, J., Wiczkowski, W. et al. If phenolic compounds in the soil with buckwheat residues affect the emergence and growth of weed seedlings?. Acta Physiol Plant 42, 154 (2020). https://doi.org/10.1007/s11738-020-03142-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03142-9