Abstract
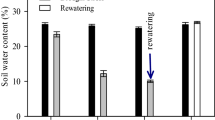
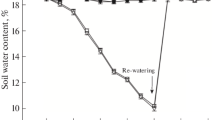
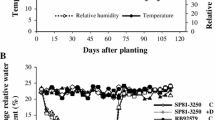
Drought is the main yield limiting factor in most sugarcane crops worldwide. This study aimed to assess the relationship between osmoregulators, photochemical efficiency of photosystem II (PSII), and regulation of electron transport rate (ETR) in sugarcane varieties under drought stress and rewatering. The experiment was conducted in pots, with two sugarcane varieties, RB72454 and RB92579. At 105 days after planting, plants were submitted to irrigation (control) and no irrigation (drought stress), followed by rewatering. The RB72454 variety was the most sensitive to drought stress, with low proline (Pro) accumulation, no change in glycine betaine (GB) concentrations, and greater electrolyte leakage (EL). High photochemical damage was also observed in PSII, including further reductions in maximum (Fv/Fm) and effective quantum efficiencies of photosystem II (ΦPSII). Drought also reduced ETR and increased non-photochemical quenching (qN), which may have contributed to delaying recovery of the RB72454 variety after rewatering. When the RB92579 variety was subjected to drought stress, it presented higher Pro and GB accumulation, lower EL and photoinhibition and small declines in Fv/Fm, ETR and ΦPSII, and slight qN increase. This resulted in less damage to the PSII photosynthetic apparatus under drought stress and contributed to maintaining cell turgidity and protecting the membrane structures of RB92579 variety chloroplasts. In addition, osmoregulators’ accumulation persisted in the RB92579 variety after rewatering, allowing for faster recovery after stress.





Similar content being viewed by others
Abbreviations
- EL:
-
Electrolyte leakage
- ETR:
-
Electron transport rate
- Fv/Fm :
-
Maximum quantum efficiency of photosystem II
- GB:
-
Glycine betaine
- Pro:
-
Proline
- PSII:
-
Photosystem II
- qN:
-
Non-photochemical quenching
- qP:
-
Photochemical quenching
- ΦPSII:
-
Effective quantum efficiency of photosystem II
References
Abbas SR, Ahmad SD, Sabir SM, Shah AH (2014) Detection of drought tolerant sugarcane genotypes (Saccharum officinarum) using lipid peroxidation, antioxidante activity, glycine-betaine and proline contentes. J Soil Sci Plant Nutrit 14:233–243
Ajithkumar IP, Panneerselvam R (2014) ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum Sumatrense Roth under drought stress. Cell Biochem Biophys 68:587–595
Amalraj RS, Selvaraj N, Veluswamy GK, Ramanujan RP, Muthurajan R, Palaniyandi M, Agrawal GK, Rakwal R, Viswanathan R (2010) Sugarcane proteomics: establishment of a protein extraction method for 2-DE in stalk tissues and initiation of sugarcane proteome reference map. Electrophoresis 31:1959–1974. https://doi.org/10.1002/elps.200900779
An Y, Zhang M, Liu G, Han R, Liang Z (2013) Proline accumulation in leaves of periploca sepium via both biosynthesis up-regulation and transport during recovery from severe drought. PLoS ONE 8:1–10. https://doi.org/10.1371/journal.pone.0069942
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Wang LC (2017) Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci 8:1–11. https://doi.org/10.3389/fpls.2017.00069
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Barbosa MHP, Resende MDV, Dias LADS, Barbosa GVS, Oliveira RAD, Peternelli LA, Daros E (2012) Genetic improvement of sugar cane for bioenergy: the Brazilian experience in network research with RIDESA. Crop Breed Appl Biotechnol 12:87–98. https://doi.org/10.1590/S1984-70332012000500010
Bates LS (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Chen THH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Chen D, Wang S, Cao B, Cao D, Leng G, Li H, Yin L, Shan L, Deng X (2016) Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front Plant Sci 6:1–15. https://doi.org/10.3389/fpls.2015.01241
Cheng Y, Deng X, Kwak S, Chen W, Eneji AE (2013) Enhanced tolerance of transgenic potato plants expressing choline oxidase in chloroplasts against water stress. Bot Stud 54:1–9. https://doi.org/10.1186/1999-3110-54-30
Cia MC, Guimarães A, Medici LO, Chabregas SM, Azevedo RA (2012) Antioxidant responses to water deficit by drought-tolerant and-sensitive sugarcane varieties. Ann Appl Biol 161:313–324. https://doi.org/10.1111/j.1744-7348.2012.00575.x
Colom MR, Vazzana V (2003) Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping lovegrass plants. Environ Exp Bot 49:135–144
Cruz FJR, Ferreira Júnior DC, Santos DMM (2018) Low salt stress affects physiological parameters and sugarcane plant growth. Aust J Crop Sci 12(8):1272–1279
Dantas Neto J, Figueirêdo JLC, Farias CHA, Azevedo HM, Azevedo CAV (2006) Resposta da canade-açúcar, primeira soca, a níveis de irrigação e adubação de cobertura. Rev Bras Eng Agríc Ambient 10:283–288
Endres L, Silva JV, Ferreira VM, Barbosa GDS (2010) Photosynthesis and water relations in Brazilian sugarcane. Open Agric J 4:31–37
Gascho GJ, Shih SF (1983) Sugarcane. In: Teare ID, Peet MM (eds) Crop-water relations. Wiley-Interscience, New York, pp 445–479
Genty B, Harbinson J, Briantais JM, Baker NR (1990) The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosynth Res 25:249–257
Ghannoum O (2008) C4 photosynthesis and water stress. Ann Bot 103:635–644
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quanternary ammonium compounds. Plant Soil 70:303–307
Guidi L, Lo Piccolo E, Landi M (2019) Chlorophyll fluorescence, photoinhibition and abiotic stress: does it make any difference the fact to be a C3 or C4 species? Front Plant Sci 10:174. https://doi.org/10.3389/fpls.2019.00174
Hemaprabha G, Swapna S, Lavanya DL, Sajitha B, Venkataramana S (2013) Evaluation of drought tolerance potential of elite genotypes and progenies of sugarcane (Saccharum sp. hybrids). Sugar Tech 15:9–16
Huang W, Fu P, Jiang Y, Zhang J, Zhang S, Hu H, Cao K (2013) Differences in the responses of photosystem I and photosystem II of three tree species Cleistanthus sumatranus, Celtis philippensis and Pistacia weinmannifolia exposed to a prolonged drought in a tropical limestone forest. Tree Physiol 33:211–220. https://doi.org/10.1093/treephys/tps132
Huseynova IM, Rustamova SM, Suleymanov SY, Aliyeva DR, Mammadov AC, Aliyev JA (2016) Drought-induced changes in photosynthetic apparatus and antioxidant components of wheat (Triticum durum Desf.) varieties. Photosynth Res 130:215–223
Jangpromma N, Thammasirirak S, Jaisil P, Songsri P (2012) Effects of drought and recovery from drought stress on above ground and root growth, and water use efficiency in sugarcane ('Saccharum officinarum' L.). Aust J Crop Sci 6:1298–1304
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102
Kato MC, Hikosaka K, Hirotsu N, Makino A, Hirose T (2003) The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol 44:318–325. https://doi.org/10.1093/pcp/pcg045
Kaur G, Asthir B (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619
Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot 103:561–579
Li R, Guo P, Baumz M, Grand S, Ceccarelli S (2006) Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric Sci China 5:751–757
Liu M, Qi H, Zhang ZP, Song ZW, Kou TJ, Zhang WJ, Yu JL (2012) Response of photosynthesis and chlorophyll fluorescence to drought stress in two maize cultivars. Afr J Agric Res 7:4751–4760
Maia Júnior SO, Andrade JR, Santos CM, Silva JAC, Santos KPO, Silva JV, Endres L (2019) Leaf thickness and gas exchange are indicators of drought stress tolerance of sugarcane. Emirates J Food Agric 31:29–38
Maxwell C, Johnson GM (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Moharramnejad S, Sofalian O, Valizadeh M, Asgari A, Shiri M (2015) Proline, glycine betaine, total phenolics and pigment contents in response to osmotic stress in maize seedlings. J Biol Sci 4:313–319. https://www.jbb.uni-plovdiv.bg
Neves JM, Aquino LA, Berger PG, Neves JC, Rocha GC, Barbosa EA (2019) Silicon and boron mitigate the effects of water deficit on sunflower. Rev Bras Eng Agríc Ambient 23:175–182. https://doi.org/10.1590/1807-1929/agriambi.v23n3p175-182
Rhein AFL, Santos DMM, Carlin SD (2011) Atividade da enzima redutase do nitrato e teores de prolina livre em raízes de cana-de-açúcar sob os estresses hídrico e ácido no solo. Semina Ciênc Agrár 32:1345–1360
Roach T, Krieger-Liszkay AK (2014) Regulation of photosynthetic electron transport and photoinhibition. Curr Protein Pept Sci 15:351–362
Sales CRG, Ribeiro RV, Silveira JAG, Machado EC, Martins MO, Lagôa AMMA (2013) Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2013.10.012
Santos CM, Silva MA (2015) Physiological and biochemical responses of sugarcane to oxidative stress induced by water deficit and paraquat. Acta Physiol Plant 37:1–14
Santos CM, Endres L, Silva ACS, Silva JV, Barbosa GVS, Froehlich A, Teixeira MM (2019) Water relations and osmolite accumulation related to sugarcane yield under drought stress in a tropical climate. Int J Plant Prod. https://doi.org/10.1007/s42106-019-00050-y
Satbhai RD, Naik RM (2014) Osmolytes accumulation, cell membrane integrity, and antioxidant enzymes in sugarcane varieties differing in salinity tolerance. Sugar Tech 16:30–35
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 49–70
Silva MA, Jifon JL, Silva JAG, Santos CM, Sharma V (2014) Relationships between physiological traits and productivity of sugarcane in response to water deficit. J Agric Sci 152:104–118. https://doi.org/10.1017/S0021859612000834
Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16:53–60
Trujillo I, Rivas M, Castrillo M (2013) Leaf recovery responses during rehydration after water deficit in two bean (Phaseolus vulgaris L.) cultivars. J Plant Interact 8:360–369. https://doi.org/10.1080/17429145.2012.754959
Van Dillewijn C (1952) Botany of sugarcane. Chronica Botanica, Waltham
Vanková R, Dobrá J, Štorchová H (2012) Recovery from drought stress in tobacco: an active process associated with the reversal of senescence in some plant parts and the sacrifice of others. Plant Signal Behav 7:19–21. https://doi.org/10.4161/psb.7.1.18375
Vantini JS, Carlin SD, Gimenez DFJ, Perecin D, Ferro JA, Ferro MIT (2016) Proline accumulation in sugarcane roots subjected to drought conditions. Científica 44:592–598. https://doi.org/10.15361/1984-5529.2016v44n4p592-598
Vitti GC, Luz PHC, Altran WS (2013). Nutrição e adubação. In: Santos F, Borém A (eds) Cana-de-açúcar: do plantio à colheita. UFV, Viçosa
Waclawovsky AJ, Sato PM, Lembke CG, Moore PH, Souza GM (2010) Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotech J 8:263–276. https://doi.org/10.1111/j.1467-7652.2009.00491.x
Wang GP, Zhang XY, Li F, Luo Y, Wang W (2010) Overaccumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica 48:117–126
Zhang C, Zhan DX, Luo HH, Zhang YL, Zhang WF (2016) Photorespiration and photoinhibition in the bracts of cotton under water stress. Photosynthetica 54:12–18
Zhang LX, Lai JH, Liang ZS, Ashraf M (2014) Interactive effects of sudden and gradual drought stress and foliar-applied glycinebetaine on growth, water relations, osmolyte accumulation and antioxidant defence systemin two maize cultivars differing in drought tolerance. J Agron Crop Sci 200:425–433. https://doi.org/10.1111/jac.12081
Zhang X, Lei L, Lai J, Zhao H, Song W (2018) Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol 18:1–16. https://doi.org/10.1186/s12870-018-1281-x
Zivcak M, Brestic M, Balatova Z, Drevenakova P, Olsovska K, Kalaji HM, Yang X, Allakhverdiev SI (2013) Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth Res 117:529–546
Acknowledgements
The authors would like to thank the National Council for Scientific and Technological (CNPq), the Coordination of Superior Level Staff Improvement (CAPES), and the Sugarcane Genetic Breeding Program (PMGCA/RIDESA/UFAL).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Oliveira Maia Júnior, S., de Andrade, J.R., dos Santos, C.M. et al. Osmoregulators’ accumulation minimizes the effects of drought stress in sugarcane and contributes to the recovery of photochemical efficiency in photosystem II after rewatering. Acta Physiol Plant 42, 62 (2020). https://doi.org/10.1007/s11738-020-03050-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03050-y