Abstract
The accumulation of soluble carbohydrates in maturing diaspores of flowering plants comprising Arctic populations of Cerastium alpinum, indigenous Antarctic species Colobanthus quitensis and Deschampsia antarctica, and cosmopolitan Poa annua from the Antarctic was investigated. For comparative purposes, the diaspores of two species of flowering plants growing in the area of Olsztyn (Poland), Poa annua (Poaceae) and Cerastium arvense (Caryophyllaceae) were used. A qualitative and quantitative analysis of soluble carbohydrates conducted by means of high-resolution gas chromatography showed that monosaccharides (glucose and fructose), maltose and sucrose, raffinose, myo-inositol and galactinol are ubiquitous in developing and mature diaspores among investigated species. Moreover, D. antarctica and P. annua caryopses additionally contained stachyose and 1-kestose; the seeds of Caryophyllaceae studied were found to contain d-pinitol and d-ononitol. The development and maturation of the seeds of polar Caryophyllaceae and Poaceae were accompanied by the changes in the concentration of their soluble carbohydrates. During maturation, seeds accumulated galactinol and raffinose family of oligosaccharides (RFOs), except C. quitensis. Although seeds of the studied Caryophyllaceae contained d-pinitol and lower amounts of d-ononitol, they did not accumulate α-d-galactoside derivatives of mentioned cyclitols. P. annua caryopses, occurring in the Antarctic, were found to accumulate considerably higher amounts of sucrose and 1-kestose than those developed in Olsztyn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soluble carbohydrates are a major group of metabolites that determine the growth and development of vegetative and generative tissues in plants. Those metabolites also supply developing embryos with carbon compounds and sources of energy (Borisjuk et al. 2003). Changes in the concentrations of hexose and sucrose that reach a developing embryo determine the success of embryogenesis, the transition from the histodifferentiation phase to the reserve accumulation phase, and the rate at which those reserves are accumulated (Borisjuk et al. 2004; Weber et al. 2005). In mature seeds, low-molecular-weight soluble carbohydrates such as sucrose and raffinose family oligosaccharides (RFOs) account for a small portion (less than 20 % of dry mass, DM) of storage reserves (Obendorf and Górecki 2012), but they are used already in the first hours of germination, which indicates that sucrose and oligosaccharides are important sources of energy during initial stages of seeds germination (Blöchl et al. 2008; Lahuta and Goszczyńska 2009). In addition to sucrose and RFOs, fructans and polyhydroxy alcohols, both non-cyclic (mannitol, sorbitol) and cyclic (myo-inositol and its isomers or methylated derivatives), increase the resistance of vegetative tissues to abiotic stressors (Valluru and Van den Ende 2011; ElSayed et al. 2014). Soluble carbohydrates contribute to embryonic resistance against drying in the final stages of development of orthodox seeds (Obendorf and Górecki 2012).
The accumulation of soluble carbohydrates in developing seeds was analyzed mostly in crop plants, including legumes (Obendorf and Górecki 2012), cereals (Lahuta and Goszczyńska 2010) and oil-bearing plants (Li et al. 2011). The highest hexose levels were reported in early stages of embryonic development when sucrose is broken down by invertase in seed coat tissues. The drop in hexose concentrations and the rise in sucrose levels in embryo induce the transfer from the histodifferentiation phase to the reserve accumulation phase (Weber et al. 1998). Sucrose concentrations are high in the middle phase of storage reserve accumulation, and they decrease gradually towards the end of seed development and embryonic desiccation. The accumulation of RFOs begins in the middle phase of storage reserve accumulation. Galactinol (α-d-galactopyranosyl-(1 → 1)-1L-myo-inositol), a major donor of galactose residues for the RFO biosynthetic pathway, is synthesized several days earlier than RFO. Embryo tissues produce raffinose (α-d-galactopyranosyl-(1 → 6)-α-d-glucopyranosyl-(1 → 2)-β-d-fructofuranoside) from sucrose and galactinol and then higher homologs of raffinose—stachyose (α-d-galactopyranosyl-(1 → 6)-α-d-galactopyranosyl-(1 → 6)-α-d-glucopyranosyl-(1 → 2)-β-d-fructofuranoside) and verbascose (α-d-galactopyranosyl-(1 → 6)-α-d-galactopyranosyl-(1 → 6)-α-d-galactopyranosyl-(1 → 6)-α-d-glucopyranosyl-(1 → 2)-β-d-fructofuranoside). This process is intensified at the end of maturation, in the phase of seed desiccation. Legume seeds accumulate the largest amounts of verbascose and stachyose (up to 10 % DM) (Obendorf and Górecki 2012). Cereal kernels store mainly raffinose (Barnes 1982; Horbowicz and Obendorf 1994; Lahuta and Goszczyńska 2010), and rapeseed—mainly stachyose and small amounts of raffinose (Li et al. 2011).
Galactinol is synthesized by galactinol synthase (GolS, EC 2.4.1.123) which catalyzes the transfer of galactose from UDP-galactose to myo-inositol. GolS is an enzyme whose activity levels are one order of magnitude higher in comparison with activities of galactosyltransferases from the RFO biosynthetic pathway (Peterbauer et al. 2001; Lahuta 2006; Lahuta et al. 2010a). Raffinose and stachyose are synthesized when galactose is transferred from galactinol to the respective acceptor, sucrose and raffinose, in the presence of raffinose synthase (RS, EC 2.4.1.82) and stachyose synthase (STS, EC 2.4.1.67), respectively. Myo-inositol is released during the transfer of galactose from galactinol. Stachyose synthase is probably also responsible for the synthesis of verbascose (Peterbauer et al. 2003).
In addition to RFOs, the seeds of selected plant species also contain α-d-galactosyl cyclitols, which differ in structure from RFOs in that sucrose is replaced by cyclitol (Obendorf et al. 2012). Those compounds are produced simultaneously with RFOs in a shared biosynthetic pathway (Peterbauer and Richter 2001). GolS can also catalyze the synthesis of mono-galactosides of d-chiro-inositol (fagopyritol A and fagopyritol B) (Obendorf et al. 2004; Ueda et al. 2005). The enzyme does not show affinity for methylated derivatives of inositol: d-pinitol and d-ononitol (Peterbauer and Richter 2001; Obendorf et al. 2004). Galactose is transferred from galactinol to the above compounds mainly by multifunctional STS (Peterbauer et al. 2002a) and, to a lesser extent, by RS (Peterbauer et al. 2002b). The rate at which galactosyl cyclitols are accumulated seems to be closely linked with the type and concentration of cyclitols that are supplied from maternal tissues to a developing embryo (Obendorf and Górecki 2012). In addition to myo-inositol, which is abundant in plant tissues (Loewus and Murthy 2000), an embryo may also be supplied with isomers (d-chiro-inositol) and methylated derivatives (d-pinitol, d-ononitol) of myo-inositol from the maternal plant. The embryo responds by producing the corresponding galactosyl cyclitols. Fagopyritols (mono-, di- and trigalactosides of d-chiro-inositol) are also synthesized in buckwheat seeds, which contain myo-inositol as well as d-chiro-inositol. Fagopyritols replace RFOs in the final stages of development (Horbowicz and Obendorf 2005). Higher levels of d-pinitol than of myo-inositol promote the synthesis of galactopinitols at the expense of RFOs in several plant species of the genus Vicia (Lahuta et al. 2005a). In developing soybeans (Gomes et al. 2004), buckwheat seeds (Ma et al. 2005; Ueda et al. 2005), vetch seeds (Lahuta et al. 2005b, c, 2010b) and peas (Lahuta et al. 2010c; Lahuta and Dzik 2011), cyclitol concentrations determine the accumulation of both types of α-d-galactosides: RFO and galactosyl cyclitols. In developing wheat kernels, where raffinose is the only accumulated RFO, small amounts of fagopyritol B1 (up to 3 mg g−1 DM) and tenfold lower amounts of galactopinitol A (galactosyl pinitol A) can be synthesized when seeds are supplied with d-chiro-inositol and d-pinitol, respectively (Lahuta and Goszczyńska 2010).
Vegetative tissues of grasses, including cereals, produce fructans—oligosaccharides where fructose residues bind with sucrose. Fructans contribute to plant resistance to low temperature and soil drought (Livingston et al. 2009; Sandve et al. 2011). In the generative phase and during kernel development, fructans synthesized and accumulated in shoots are remobilized to supply kernels with additional sucrose (Amiard et al. 2003; Zhang et al. 2015). Fructans (like 1-kestose) present in immature kernels are used up during starch synthesis during reserve accumulation (Verspreet et al. 2013; Peukert et al. 2014). In mature cereal kernels, 1-kestose (α-d-glucopyranosyl-(1 → 2)-β-d-fructofuranoside-(1 → 2)-β-d-fructofuranoside) may be the second most abundant soluble carbohydrate after sucrose (Lahuta and Goszczyńska 2010).
The final composition and content of soluble carbohydrates in mature seeds are determined not only by genetic factors, but also by environmental stresses that disrupt the accumulation of storage reserves. Soil drought during seed maturation can stimulate the accumulation of RFOs and sucrose, which was demonstrated in faba beans (Lahuta et al. 2000), or can lower the accumulation of soluble carbohydrates, which was reported in yellow lupine seeds and triticale kernels (Zalewski et al. 2001). In yellow lupine seeds, the above was accompanied by changes in oligosaccharide proportions with an increase in the content of verbascose, mono- and di-galactosides of d-pinitol. The increase in galactopinitols levels could be attributed to a rise in d-pinitol concentrations in vegetative tissues in response to water stress, which was observed in a study of soybeans (Streeter et al. 2001), and, in consequence, intensified transfer of d-pinitol to developing seeds.
Very little is known about the accumulation, composition and content of soluble carbohydrates in the seeds of plants that inhabit extreme climates. The composition and content of soluble carbohydrates in vegetative tissues of several vascular plants growing in the Earth’s polar regions were analyzed in our previous study (Pastorczyk et al. 2014). An effective method for vegetative propagation of several species of vascular plants from the Arctic (Cerastium alpinum and Poa arctica var. vivipara) and the Antarctic (Colobanthus quitensis and Deschampsia antarctica) was proposed to investigate the rate of changes in the composition and content of soluble carbohydrates in shoots under short-term exposure to cold stress (Pastorczyk et al. 2014). High levels of d-pinitol (threefold to sixfold higher than sucrose concentrations) and RFOs as well as small amounts of galactopinitols were determined in C. quitensis and C. alpinum. All plants accumulated larger amounts of sucrose in response to cold stress. An increase in RFO concentrations was noted in Caryophyllaceae, whereas an increase in 1-kestose levels was observed in Poaceae plants (Pastorczyk et al. 2014).
During the reproductive phase, cold stress contributes to metabolic and developmental disorders that lead to withering of flowers, abnormal pollination and fertilization, abnormal embryonic development and impaired seed development (Thakur et al. 2010). Vascular plants inhabiting Arctic region produce small amounts of seeds, many of which are nonviable. The above is particularly visible in growing seasons with low temperatures (Phillip et al. 1990). Flowers and fruit are more abundant when weather conditions are more favorable, which happens every few years or even every ten or more years (Pirożnikow 1993). Plant species native to the Antarctic produce numerous flowers and inflorescences nearly every year, but mature seeds do not emerge regularly on an annual basis (Convey 1996; Giełwanowska et al. 2011).
The described fluctuations and low seed production rates in polar plants as well as the low availability of experimental material probably explains the absence of research into the physiology of polar plant seeds and changes in the chemical composition of developing seeds. The objective of this study was the comparison of changes in the content and composition of soluble carbohydrates during seed development of several species of Arctic and Antarctic flowering plants: C. quitensis and C. alpinum of the family Caryophyllaceae, and Deschampsia antarctica and Poa annua of the family Poaceae. The seeds of Cerastium arvense and Poa annua plants harvested in the area of Olsztyn were included in the study for comparative purposes.
Materials and methods
Materials
The experimental material comprised four species of polar vascular plants belonging to the families Caryophyllaceae and Poaceae. Cerastium alpinum L. (Caryophyllaceae) was harvested from the Arctic. Two species of vascular plants native to the Antarctic, Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) and Deschampsia antarctica Desv. (Poaceae), as well as the cosmopolitan species of Poa annua L. (Poaceae) were harvested in the Antarctic. The seeds of two species of popular flowering plants in Poland, Poa annua (Poaceae) and Cerastium arvense L. (Caryophyllaceae), were analyzed for comparative purposes.
Seeds for analyses of the content and composition of soluble carbohydrates were harvested in 2010–2012 from plants grown in a greenhouse and from naturally growing plants (Table 1). C. alpinum and C. quitensis seeds were harvested from plants grown in the experimental greenhouse of the University of Warmia and Mazury in Olsztyn (53°76′N and 20°46′E), whereas seeds of the remaining 3 species, C. arvense, D. antarctica and P. annua, were harvested from plants growing in their natural habitats. Greenhouse-grown specimens of C. alpinum and C. quitensis were grown from seeds harvested in the region of the Stanisław Siedlecki Polish Polar Station in Hornsund, Spitsbergen (Svalbard Archipelago, 77°00′N and 15°33′E) and the Henryk Arctowski Polish Antarctic Station on King George Island (South Shetland Islands, 62°09′S and 58°28′W), respectively. The content and composition of soluble carbohydrates were determined in developing and maturing seeds collected at three developmental stages (Table 2). The examined seeds were very small (Table 3) and they were characterized by a high share of seed coat tissue (Fig. 1), therefore whole diaspores were used in the analysis.
Immediately after harvest, seeds were microwaved (350 W, 2 min) to deactivate enzymes, and they were dried in a hot air oven at the temperature of 80 °C. Dried material was pulverized in a vibrating mill (Retsch MM200) for 2 min at the frequency of 22 Hz. Samples of pulverized material (10–50 mg each, in 3 replications, subject to availability) were collected for extraction of soluble carbohydrates.
Analysis of soluble carbohydrates
The composition and content of soluble carbohydrates were analyzed by gas chromatography according to the method described earlier (Lahuta 2006). Carbohydrates were extracted with 50 % aqueous ethanol solution containing an internal standard (xylitol). The samples were heated at 90 °C for 30 min and centrifuged. A portion of the homogenate was deionized by shaking with a mixture of Dowex ion exchange resins, and concentrated until dry. Sediments were derived with a mixture of TMSI (trimethylsilyl-imidazole) and pyridine (1:1, v/v). TMS derivatives of carbohydrates were separated in a ZEBRON ZB-1 chromatographic column (15 m × 0.25 mm, active layer of 100 % dimethyl polysiloxane with the thickness of 0.1 µm, Phenomenex, USA) in the Shimadzu GC 2010 gas chromatograph (Japan). Chromatograms were analyzed with an integrator in the CHROMA 3.2 application (Pol-Lab, Poland). Chromatographic peaks were identified by comparing their retention times with the retention time of the standard. The content of the analyzed carbohydrates was calculated by the internal standard method.
Statistical analysis
The results were presented as means from three replications with standard error (SE). The significance of differences in the concentrations of various carbohydrates was determined by one-way ANOVA with Tukey’s test. Calculations were performed separately for every compound at the significance level of p < 0.05. Data were processed in the Statistica v.10 program (StatSoft, Poland).
Results
Mature seeds of Caryophyllaceae plants differed significantly in size and weight (Table 3) from 0.043 (C. quitensis) to 0.294 mg seed−1 (C. alpinum). Diaspores of C. alpinum and C. arvense had extensive seed coats (Fig. 1b, c). Poaceae kernels were characterized by similar habit, variations in the length-to-width ratio and smaller differences in weight (from 0.226 in Deschampsia antarctica to 0.321–0.336 mg seed−1 in Poa annua) than Caryophyllaceae seeds (Table 3). C. quitensis and D. antarctica had the lightest diaspores.
Composition and content of soluble carbohydrates in mature seeds
Caryophyllaceae
In mature seeds of C. quitensis, C. alpinum and C. arvense plants, the content of soluble carbohydrates was determined at 3–4 % DM. Soluble carbohydrates were composed of monosaccharides (glucose and fructose), disaccharides (sucrose and maltose), raffinose, cyclitols (myo-inositol, d-pinitol and d-ononitol) and galactinol (Table 4). Sucrose was the predominant soluble carbohydrate. The largest amounts of sucrose were observed in C. quitensis seeds (23.65 mg g−1 DM), and the smallest amounts were noted in C. arvense seeds (18.89 mg g−1 DM) (Table 4). The percentage of sucrose in total soluble carbohydrates (TSC) ranged from 45.92 % (C. arvense) to 71.73 and 76.13 % (C. alpinum and C. quitensis, respectively). Monosaccharides accounted for 1–5.6 % of TSC. Fructose and glucose concentrations were lowest in C. quitensis seeds and highest in C. arvense seeds. The maltose content of C. quitensis and C. alpinum seeds was similar to their monosaccharide content, but it was surprisingly high at 39.5 % TSC in C. arvense (Table 4). Raffinose, the only detected RFO, accounted for 0.01–0.5 % DM of seeds. The seeds of all three Caryophyllaceae species also contained small amounts of myo-inositol and d-pinitol. Trace amounts of d-ononitol were detected in C. quitensis seeds. Despite higher concentrations of d-pinitol than myo-inositol, d-pinitol α-d-galactosides were not found. Galactinol was the only galactosyl cyclitol that occurred abundantly in the evaluated seeds. The largest amounts of galactinol were detected in C. alpinum seeds (4.12 mg g−1 DM), and the smallest amounts were noted in C. quitensis seeds (0.13 mg g−1 DM).
Poaceae
Mature Poaceae kernels contained monosaccharides (glucose and fructose), disaccharides (sucrose and maltose), oligosaccharides (maltotriose, raffinose and stachyose), myo-inositol, galactinol and 1-kestose (Table 5). Soluble carbohydrates accounted for 3.2–6.6 % DM in the kernels of D. antarctica and P. annua plants harvested in the Antarctic (Table 5). The kernels of P. annua harvested in the area of Olsztyn were less abundant in soluble carbohydrates (1.8 % DM). Similarly to the kernels of P. annua plants grown in Olsztyn, D. antarctica kernels were characterized by very low concentrations of monosaccharides (1.7–1.8 % of TSC), whereas P. annua kernels from the Antarctic contained more monosaccharides (21.4 % of TSC) (Table 5). Sucrose was the predominant soluble carbohydrate in D. antarctica and P. annua. Sucrose concentrations in P. annua kernels that developed in the Antarctic were nearly threefold higher than in P. annua kernels that developed in Olsztyn and 1.5-fold higher than in D. antarctica kernels. P. annua kernels from the Antarctic were also characterized by higher levels of fructan—1-kestose (Table 5). The concentrations of myo-inositol and galactinol were lower than the levels of sucrose and 1-kestose, but they were comparable in D. antarctica and P. annua kernels that developed in Olsztyn and in the Antarctic. RFOs were represented by raffinose and stachyose (Table 5). Poaceae kernels contained smaller amounts of RFOs (0.04–1.69 mg g−1 DM) than Caryophyllaceae seeds (0.16–5.36 mg g−1 DM) (Table 4).
Changes in the content and composition of soluble carbohydrates during seed development and maturation
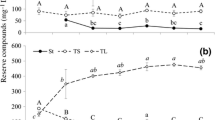
Similar changes in sucrose, monosaccharide and myo-inositol concentrations were observed in maturing seeds of Caryophyllaceae and Poaceae plants (Figs. 2a–c, g–i, 3a–f). Sucrose concentrations decreased steadily in C. alpinum and C. arvense seeds (Fig. 2b, c) and in D. antarctica and P. annua seeds (Fig. 3a–c). Sucrose levels remained stable only in C. quitensis seeds (Fig. 2a). The drop in sucrose content was accompanied by a decrease in the levels of monosaccharides (Caryophyllaceae, Fig. 2a–c; D. antarctica and P. annua harvested in Olsztyn area, data not presented), maltose, maltotriose (Poaceae, Fig. 4d–f) and myo-inositol (Figs. 2g–i, 3d–f). During seed maturation, fructose and glucose content increased only in P. annua kernels harvested in the Antarctic (from 3.91 and 2.77 mg g−1 DM in phase D1 to 10.32 and 3.79 mg g−1 DM in phase D3). In developing Caryophyllaceae seeds, maltose concentrations increased in C. quitensis and C. arvense (Fig. 4a, c), but decreased in C. alpinum (Fig. 4b). A steady decrease in d-pinitol (Fig. 2g–i) and d-ononitol (data not presented) concentrations in Caryophyllaceae seeds, and a reduction in 1-kestose levels in Poaceae seeds (Fig. 3a–c) were observed between phases D1 and D3.
Changes in the concentration of monosaccharides, sucrose (a–c), galactinol, raffinose (d–f), myo-inositol and d-pinitol (g–i) in developing and maturing seeds of Colobanthus quitensis (a, d, g), Cerastium alpinum (b, e, h) and Cerastium arvense (c, f, i). Developmental stages: D1—terminated cell divisions, D2—intensive accumulation of storage materials, D3—mature seeds (see also Table 2). Mean ± SE (n = 3). The averages for a particular compound marked with the same letter are not different significantly (Tukey test, p < 0.05)
Changes in the concentration of sucrose, 1-kestose (a–c), galactinol, myo-inositol (d–f), raffinose and stachyose (g–i) in developing and maturing grains of Deschampsia antarctica (a, d, g), Poa annua collected in the Antarctic (b, e, h) and in the vicinity of Olsztyn (c, f, i). Developmental stages: D1—milk-ripeness stage, D2—wax-ripeness stage, D3—full-ripeness stage. Mean ± SE (n = 3). The averages for a particular compound marked with the same letter are not different significantly (Tukey test, p < 0.05)
Changes in the concentration of maltose and maltotriose in developing and maturing seeds of Colobanthus quitensis (a), Cerastium alpinum (b), Cerastium arvense (c), and grains of Deschampsia antarctica (d) and Poa annua collected in the Antarctic (e) and in the vicinity of Olsztyn (f). Mean ± SE (n = 3). The averages for a particular compound marked with the same letter are not different significantly (Tukey test, p < 0.05)
Maturing seeds of both plant families accumulated galactinol and raffinose, and D. antarctica and P. annua kernels harvested in the Antarctic also stored stachyose, but the rate at which those compounds were accumulated differed between the analyzed species. In phase D1, the seeds of all evaluated taxa contained small amounts of galactinol (Figs. 2d–f, 3d–f). Galactinol concentrations increased steadily during seed maturation, except for C. quitensis where galactinol levels was significantly reduced between phases D2 and D3. The analyzed seeds also accumulated raffinose (Figs. 2d–f, 3g–i) whose concentrations increased particularly at the end of seed maturation in the desiccation phase (between phases D2 and D3). Unlike in other species, the raffinose content of C. quitensis and C. alpinum seeds decreased in the desiccation phase (Fig. 2d, e). D. antarctica and P. annua kernels harvested in the Antarctic produced stachyose during desiccation (Fig. 3g, h). The predominant RFOs were raffinose in mature D. antarctica seeds (Fig. 3g) and stachyose in mature P. annua seeds (Fig. 3h). Stachyose was not detected in P. annua kernels from the Olsztyn area.
Discussion
Low concentrations of soluble carbohydrates in mature seeds of Caryophyllaceae (3.1–4.1 % DM) and Poaceae (1.7–6.6 % DM) plants indicate that, similarly to the observations made in crop plants, (Obendorf and Górecki 2012), soluble carbohydrates had a small share of storage reserves in the evaluated taxa. Similarly to cereal kernels (Horbowicz and Obendorf 1994; Lahuta and Goszczyńska 2010), sucrose was the predominant soluble carbohydrate (70–90 % TSC, in C. arvense—45 % TSC), and RFOs accounted for only 0.4–17.3 % TSC (Tables 4, 5). Changes in the concentrations of sucrose, myo-inositol, d-pinitol (Caryophyllaceae) and 1-kestose (Poaceae) in maturing seeds of Caryophyllaceae and Poaceae plants were similar to those observed in crop plants (Obendorf and Górecki 2012). The highest content of the above carbohydrates was observed in initial stages of reserve accumulation in phase D1 (Table 2). Their concentrations decreased steadily during seed maturation (Figs. 2a–c, g–i, 3a–f).
A significant increase in monosaccharide concentrations (mainly fructose) was observed for the first time at the end of maturation in P. annua seeds harvested in the Antarctic. The above could be attributed to the breakdown of fructans accumulated in vegetative tissues and/or tissues covering the embryo and endosperm. The fructan content of timothy and ryegrass increased between October and January (Østrem et al. 2011). In the shoots and leaves of winter wheat, fructan levels increased during cold acclimation and decreased in late winter and in spring (Yoshida et al. 1998). Changes in fructan concentrations in vegetative tissues of polar plants during the growing season have never been investigated. In our previous study, short-term exposure to cold stress stimulated the accumulation of 1-kestose in shoots of D. antarctica and Poa arctica plants (Pastorczyk et al. 2014). Peukert et al. (2014) described changes in the composition, content and location of soluble carbohydrates, including fructans, in maturing barley kernels. They demonstrated that oligofructans determine the sink strength of developing and maturing kernels, which improves the supply of nutrients to the embryo and endosperm. Developmental disorders, such as seed coat wrinkling due to lower starch accumulation, were not observed in mature seeds of P. annua (Fig. 1e). The causes and physiological significance of high fructose levels in mature P. annua seeds have not been elucidated. High levels of maltose in mature seeds of C. arvense (Table 4) are also difficult to explain. Maltose concentrations initially decreased (between phases D1 and D2) in the seeds of both investigated plant families, but a rapid increase in maltose levels in the desiccation phase was observed only in C. arvense (Fig. 4c), which points to the activation of starch hydrolyzing enzymes.
In maturing seeds, galactinol accumulation determines the biosynthesis of RFOs (Obednorf and Górecki 2012). Galactinol concentrations were initially high in maturing soybeans (Saravitz et al. 1987), lupine seeds (Górecki et al. 1997), peas (Górecki et al. 2000), vetch seeds (Lahuta et al. 2005a) and rapeseed (Li et al. 2011), but they decreased rapidly towards the end of RFO accumulation. In the evaluated plants, similar changes in the galactinol content of seeds were observed only in C. quitensis (Fig. 2d). The remaining species accumulated increasingly more galactinol, despite the accumulation of raffinose, and Poaceae plants also stored stachyose (Figs. 2e, f, 3d–i). The RFO content of mature plants was very low (<5.4 mg g−1 DM). The absence of stachyose in Caryophyllaceae and verbascose in Poaceae could imply that STS is not expressed in Caryophyllaceae and that it is unable to synthesize verbascose in Poaceae. The absence of verbascose could also be attributed to insufficient levels of stachyose, a substrate for verbascose synthesis. Enzyme responsible solely for verbascose synthesis have not been identified in seeds to date, and it is believed that verbascose synthesis is catalyzed by STS (Peterbauer et al. 2003). Mutations in the STS gene are probably responsible for the absence of verbascose or variations in its levels (Jones et al. 1999; Górecki et al. 2000). Stachyose synthase also catalyzes synthesis of galactopinitols in seeds that accumulate d-pinitol (Peterbauer and Richter 2001). In Caryophyllaceae, the absence of STS activity probably prevented the accumulation of galactopinitols despite high initial concentrations of d-pinitol. Lower concentrations of RFOs in mature seeds of locally grown C. arvense and P. annua plants than in the seeds of greenhouse-grown plants (C. quitensis and C. alpinum) and plants harvested in the Antarctic (D. antarctica and P. annua) could point to differences in gene expression and activity levels of GolS, RS and STS during seed maturation.
Changes in the expression of genes encoding the RFO biosynthetic pathway were described in peas (Peterbauer et al. 2001), corn (Zhao et al. 2004a) and rapeseed (Li et al. 2011), and changes in enzyme activity were also characterized in developing soybeans (Saravitz et al. 1987) and seeds produced by plants of the genus Vicia (Lahuta 2006; Lahuta et al. 2010a, 2010b). Higher levels of GolS expression and GolS activity are characteristic of the middle phase of reserve accumulation, but they were found to decrease in soybeans (Saravitz et al. 1987), peas (Peterbauer et al. 2001) and vetch (Lahuta 2006), and increase in corn (Zhao et al. 2004a) in successive stages of seed maturation. Accelerated desiccation of mature corn seeds increases ZmGOLS expression and GolS activity (Zhao et al. 2004b). The expression of ZmGOLS2, which encodes tissue responses to dehydration stress, is positively correlated with myo-inositol concentrations (Zhao et al. 2004b). Numerous copies of GolS genes, which were detected in vegetative tissues of Arabidopsis thaliana (Nishizawa et al. 2008), Salvia miltiorrhiza (Wang et al. 2012) and Coffea arabica (Dos Santos et al. 2011), are specifically expressed in response to abiotic stressors such as salinification, cold, dehydration and soil drought. Increased expression of GolS genes intensifies the accumulation of galactinol and raffinose in tissues and increases plant resistance to stress (Wang et al. 2012; Zhuo et al. 2013). In a study of transgenic plants with overexpression of the GolS gene, intensified accumulation of galactinol and raffinose increased resistance to cold stress in Arabidopsis (Zuther et al. 2004; Sun et al. 2013), Photinia serrulata (Song et al. 2013) and monocot Brachypodium distachyon (Himuro et al. 2014). It remains unknown whether galactinol exerts a protective influence on dehydrated embryonic tissues.
Mature seeds of C. alpinum, C. arvense and P. annua contained more galactinol than RFOs (Tables 4, 5). The existing research demonstrated that RFOs have been nearly completely replaced by galactosyl cyclitols only in buckwheat seeds (Fagopyrum esculentum) which produce α-d-galactosides of d-chiro-inositol, i.e. fagopyritols (Horbowicz and Obendorf 2005). In buckwheat, the predominant α-d-galactoside is fagopyritol B1 which is synthesized in the presence of GolS (Ueda et al. 2005). In developing buckwheat seeds, raffinose and stachyose are accumulated only until the desiccation phase, after which their concentrations decrease (Horbowicz and Obendorf 2005). In developing seeds of C. arvense, D. antarctica and P. annua, the gradual accumulation of galactinol was accompanied by a rise in RFO levels (Figs. 2f, 3d–i). In the above species, lower RFO levels than galactinol concentrations could be attributed to low activity and/or low expression of enzymes throughout the entire period of embryonic development.
Conclusion
Mature seeds of five investigated species, beside common soluble carbohydrates (glucose, fructose, sucrose, maltose, myo-inositol, galactinol, raffinose and stachyose), contain also methylated derivatives of myo-inositol (d-pinitol and d-ononitol) characteristic for Caryophyllaceae and 1-kestose, fructan characteristic for Poaceae. The common changes in carbohydrate during seed development and maturation include (1) gradually decrease in the content of sucrose, cyclitols, 1-kestose (in Poaceae), monosaccharides (except P. annua kernels harvested in the Antarctic), maltose and maltotriose (in Poaceae and C. alpinum) and (2) accumulation of galactinol and raffinose. The absence of galactosyl pinitols or galactosyl cyclitols in seeds Caryophyllaceae suggest that it can be a result of low activity and/or different catalytic properties of enzymes of RFO biosynthetic pathway. The low level of accumulated galactinol and raffinose seems to exclude protective properties of mentioned sugars in seeds of investigated species. Additionally, the extremely high concentration of fructose in mature caryopses of P. annua, harvested in the Antarctic, can be a metabolic future indicating the readiness of caryopses to start of germination in short period of favorable growing conditions.
Author contribution statement
Wioleta Kellmann-Sopyła (Ph.D.): data acquisition and analysis (plant cultivation, seed collection and preparation for analyses, analysis of soluble carbohydrates by gas chromatography method, biometric measurements, statistical analysis of data) and drafting of manuscript (main text, figures and tables; references). Prof. Lesław B. Lahuta: revision of the results obtained by gas chromatography method, presentation design of the results, and discussion of results. Irena Giełwanowska (Ph.D.): research concept and experiment design, discussion of results, and manuscript preparation. Prof. Ryszard J. Górecki: discussion of results.
References
Amiard V, Morvan-Bertrand A, Billard J-P, Huault C, Keller F, Prud’homme MP (2003) Fructans, but not the sucrosyl-galactosides, raffinose and loliose, are affected by drought stress in perennial ryegrass. Plant Physiol 132:2218–2229. doi:10.1104/pp.103.022335
Barnes PJ (1982) Composition of cereal germ preparations. Z Lebensm Unters Forsch 174:467–471
Blöchl A, Peterbauer T, Hofmann J (2008) Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta 228:99–110. doi:10.1007/s00425-008-0722-4
Borisjuk L, Rolletschek H, Wobus U, Weber H (2003) Differentiation of legume cotyledons as related to metabolic gradients and assimilate transport into seeds. J Exp Bot 54(382):503–512. doi:10.1093/jxb/erg051
Borisjuk L, Rolletschek H, Radchuk R, Weschke W, Wobus U, Weber H (2004) Seed development and differentiation: a role for metabolic regulation. Plant Biol 6:375–386. doi:10.1055/s-2004-817908
Convey P (1996) Reproduction of Antarctic flowering plants. Antarct Sci 8:127–134. doi:10.1017/S0954102096000193
Dos Santos TB, Budzinski IG, Marur CJ, Petkowicz CL, Pereira LF, Vieira LG (2011) Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiol Biochem 49:441–448. doi:10.1016/j.plaphy.2011.01.023
ElSayed AI, Rafudeen MS, Golldack D (2014) Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol 16:1–8. doi:10.1111/plb.12053
Giełwanowska I, Bochenek A, Gojło E, Górecki R, Kellmann W, Pastorczyk M, Szczuka E (2011) Biology of reproduction of Colobanthus quitensis (Kunth) Bartl. Pol Polar Res 32(2):139–155. doi:10.2478/v10183-011-0008-6
Gomes CI, Obendorf RL, Horbowicz M (2004) myo-Inositol, d-chiro-inositol, and d-pinitol synthesis, transport, and galactoside formation in soybean explants. Crop Sci 45(2):1312–1319. doi:10.2135/cropsci2004.0247
Górecki RJ, Piotrowicz-Cieślak AI, Lahuta LB, Obendorf RL (1997) Soluble carbohydrates in desiccation tolerance of yellow lupin seeds during maturation and germination. Seed Sci Res 7:107–115. doi:10.1017/S0960258500003445
Górecki RJ, Lahuta LB, Hedley C, Jones A (2000) Soluble sugars in maturing pea seeds of different lines in relation to desiccation tolerance. In: Black M, Bradford KJ, Vasquez-Ramos J (eds) Seed Biology: advances and applications. Proceedings of the Sixth International Workshop on Seeds. CAB International, Merida, pp 67–74
Himuro Y, Ishiyama K, Mori F, Gondo T, Takahashi F, Shinozaki K, Kobayashi M, Akashi R (2014) Arabidopsis galactinol synthase AtGolS2 improves drought tolerance in the monocot Brachypodium distachyon. J Plant Physiol 171:1127–1131. doi:10.1016/j.jplph.2014.04.007
Horbowicz M, Obendorf RL (1994) Seed desiccation tolerance and storability: dependence on flatulence-producing oligosaccharides and cyclitols: review and survey. Seed Sci Res 4:385–405. doi:10.1017/S0960258500002440
Horbowicz M, Obendorf RL (2005) Fagopyritol accumulation and germination of buckwheat seeds matured at 15, 22 and 30 °C. Crop Sci 45:1264–1270
Jones DA, DuPont MS, Ambrose MJ, Frias J, Hedley CL (1999) The discovery of compositional variation for the raffinose family of oligosaccharides in pea seeds. Seed Sci Res 9:305–310. doi:10.1017/S0960258599000318
Lahuta LB (2006) Biosynthesis of raffinose family oligosaccharides and galactosyl pinitols in developing and maturing seeds of winter vetch (Vicia villosa Roth.). Acta Soc Bot Pol 75(3):219–227. doi:10.5586/asbp.2006.026
Lahuta LB, Dzik T (2011) d-chiro-Inositol affects accumulation of raffinose family oligosaccharides in developing embryos of Pisum sativum. J Plant Physiol 168:352–358. doi:10.1016/j.jplph.2010.07.027
Lahuta LB, Goszczyńska J (2009) Inhibition of raffinose family oligosaccharides and galactosyl pinitols breakdown delays germination of winter vetch (Vicia villosa Roth.) seeds. Acta Soc Bot Pol 78(3):203–208. doi:10.5586/asbp.2009.025
Lahuta LB, Goszczyńska J (2010) Cyclitols in maturing grains of wheat, triticale and barley. Acta Soc Bot Pol 79(3):181–187. doi:10.5586/asbp.2010.023
Lahuta LB, Łogin A, Rejowski A, Socha A, Zalewski K (2000) Influence of water deficit on the accumulation of sugars in developing field bean (Vicia faba var. minor) seeds. Seed Sci Technol 28:93–100
Lahuta LB, Górecki RJ, Gojło E, Horbowicz M (2005a) Differences in accumulation of soluble a-d-galactosides during seed maturation of several Vicia species. Acta Physiol Plant 27(2):163–171. doi:10.1007/s11738-005-0020-8
Lahuta LB, Górecki RJ, Horbowicz M (2005b) High concentrations of d-pinitol or d-chiro-inositol inhibit the biosynthesis of raffinose family oligosaccharides in maturing smooth tare (Vicia tetrasperma [L.] Schreb.) seeds. Acta Physiol Plant 27(4A):505–513. doi:10.1007/s11738-005-0056-9
Lahuta LB, Horbowicz M, Gojło E, Goszczyńska J, Górecki RJ (2005c) Exogenously applied d-pinitol and d-chiro-inositol modifies the accumulation of a-d-galactosides in developing tiny vetch (Vicia hirsuta [L.] S.F. Gray) seeds. Acta Soc Bot Pol 74(4):287–296. doi:10.5586/asbp.2005.037
Lahuta LB, Goszczyńska J, Horbowicz M (2010a) Seeds a-d-galactosides of selected Vicia species and enzymes involved in their biosynthesis. Acta Biol Cracov Bot 52(1):27–35. doi:10.2478/v10182-010-0004-x
Lahuta LB, Goszczyńska J, Horbowicz M, Hołdyński C, Górecki RJ (2010b) Cyclitols affect accumulation of a-d-galactosides in developing Vicia seeds. Acta Physiol Plant 32:933–942. doi:10.1007/s11738-010-0481-2
Lahuta LB, Święcicki W, Dzik T, Górecki RJ, Horbowicz M (2010c) Feeding stem-leaf-pod explants of pea (Pisum sativum L.) with d-chiro-inositol or d-pinitol modifies composition of a-d-galactosides in developing seeds. Seed Sci Res 20((4):213–221. doi:10.1017/S096025851000022X
Li X, Zhuo JJ, Jing Y, Liu X, Wang XF (2011) Expression of a GALACTINOL SYNTHASE gene is positively associated with desiccation tolerance of Brassica napus seeds during development. J Plant Physiol 168:1761–1770. doi:10.1016/j.jplph.2011.04.006
Livingston DP, Hincha DK, Heyer AG (2009) Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci 66(13):2007–2023. doi:10.1007/s00018-009-0002-x
Loewus FA, Murthy PPN (2000) myo-Inositol metabolism in plants. Plant Sci 150:1–19. doi:10.1016/S0168-9452(99)00150-8
Ma JM, Horbowicz M, Obendorf RL (2005) Cyclitol galactosides in embryos of buckwheat stem-leaf-seed explants fed d-chiro-inositol, myo-inositol or d-pinitol. Seed Sci Res 15:329–338. doi:10.1079/SSR2005221
Nishizawa A, Yabuta Y, Shigeoka S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 147:1251–1263. doi:10.1104/pp.108.122465
Obendorf RL, Górecki RJ (2012) Soluble carbohydrates in legume seeds. Seed Sci Res 22:219–242. doi:10.1017/S0960258512000104
Obendorf RL, Odorcic S, Ueda T, Coseo MP, Vasallo E (2004) Soybean galactinol synthase forms fagopyritol B1 but not galactopinitols: substrate feeding of isolated embryos and heterologous expression. Seed Sci Res 14:321–333. doi:10.1079/SSR2004186
Obendorf RL, Horbowicz M, Lahuta LB (2012) Characterization of sugars, cyclitols and galactosyl cyclitols in seeds by GC. In: Preedy V (ed) Food and nutritional compounds in focus No 3. dietary sugars: chemistry, analysis, function and effects. King’s College London, Royal Society of Chemistry Publishing, pp 167–185
Østrem L, Rapacz M, Jørgensen M, Höglind M (2011) Effect of developmental stage on carbohydrate accumulation patterns during winter of timothy and perennial ryegrass. Acta Agric Scandinavica Ser B Soil Plant Sci 61:153–163. doi:10.1080/09064711003652522
Pastorczyk M, Giełwanowska I, Lahuta LB (2014) Changes in soluble carbohydrates in polar Caryophyllaceae and Poaceae plants in response to chilling. Acta Physiol Plant 36:1771–1780. doi:10.1007/s11738-014-1551-7
Peterbauer T, Richter A (2001) Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Sci Res 11:185–197. doi:10.1079/SSR200175
Peterbauer T, Lahuta LB, Blöchl A, Mucha J, Jones DA, Hedley CL, Górecki RJ, Richter A (2001) Analysis of the raffinose family oligosaccharide pathway in pea seeds with contrasting carbohydrate composition. Plant Physiol 127:1764–1772. doi:10.1104/pp.010534
Peterbauer T, Mach L, Mucha J, Richter A (2002a) Functional expression of a cDNA encoding pea (Pisum sativum L.) raffinose synthase, partial purification of the enzyme from maturing seeds, and steady-state kinetic analysis of raffinose synthesis. Planta 215:839–846. doi:10.1007/s00425-002-0804-7
Peterbauer T, Mucha L, Mach L, Richter A (2002b) Chain elongation of raffinose in pea seeds. Isolation, characterization and molecular cloning of a multifunctional enzyme catalyzing the synthesis of stachyose and verbascose. J Biol Chem 277:194–200. doi:10.1074/jbc.M109734200
Peterbauer T, Karner U, Mucha J, Mach L, Jones DA, Hedley CL, Richter A (2003) Enzymatic control of the accumulation of verbascose in pea seeds. Plant Cell Environ 26:1385–1391. doi:10.1046/j.0016-8025.2003.01063.x
Peukert M, Thiel J, Peshev D, Weschke W, Van den Ende W, Mock HP, Matros A (2014) Spatio-temporal dynamics of fructans metabolism in developing barley grains. Plant Cell 26(9):3728–3744. doi:10.1105/tpc.114.130211
Philipp M, Böcher J, Matsson O, Woodell SRJ (1990) A quantitative approach to the sexual reproductive biology and population structure in some arctic flowering plants Dryas integrifolia, Silene aucalis. Ranunculus nivalis, Meddeletser om Grønland, Bioscience 34
Pirożnikow E (1993) Populations of Saxifraga oppositifolia L., in Spitsbergen tundra in different ecological conditions. Pol. Polar Res 14(4):355–382
Sandve SR, Kosmala A, Rudi H, Fjellheim S, Rapacz M, Yamada T, Rognli OA (2011) Molecular mechanisms underlying frost resistance in perennial grasses adapted to cold climates. Plant Sci 180:69–77. doi:10.1016/j.plantsci.2010.07.011
Saravitz DM, Pharr DM, Carter TE Jr (1987) Galactinol synthase activity and soluble sugars in developing seeds of four soybean genotypes. Plant Physiol 83:185–189
Song J, Liu J, Weng M, Huang Y, Luo L, Cao P, Sun H, Liu J, Zhao J, Feng D, Wang B (2013) Cloning of galactinol synthase gene from Ammopiptanthus mongolicus and its expression in transgenic Photinia serrulata plants. Gene 513:118–127. doi:10.1016/j.gene.2012.10.058
Streeter JG, Lohnes DG, Fioritto RJ (2001) Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance. Plant, Cell Environ 24:429–438. doi:10.1046/j.1365-3040.2001.00690.x
Sun Z, Qi X, Wang Z, Li P, Wu Ch, Zhang H, Zhao Y (2013) Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol Biochem 69:82–89. doi:10.1016/j.plaphy.2013.04.009
Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H (2010) Cold stress effects on reproductive development in grain crops: an overview. Environ Exp Bot 37:429–443. doi:10.1016/j.envexpbot.2009.09.004
Ueda T, Coseo MP, Harrell TJ, Obendorf RL (2005) A multifunctional galactinol synthase catalyzes the synthesis of fagopyritol A1 and fagopyritol B1 in buckwheat seed. Plant Sci 168:681–690. doi:10.1016/j.plantsci.2004.09.029
Valluru R, Van den Ende W (2011) myo-Inositol and beyond - emerging networks under stress. Plant Sci 181:387–400. doi:10.1016/j.plantsci.2011.07.009
Verspreet J, Cimini S, Vergauwen R, Dornez E, Locato V, Le Roy K, De Gara L, Den Van, Ende W, Delcour JA, Courtin CM (2013) Fructan metabolism in developing wheat (Triticum aestivum L.) kernels. Plant Cell Physiol 54(12):2047–2057. doi:10.1093/pcp/pct144
Wang D, Yao W, Song Y, Liu W, Wang Z (2012) Molecular characterization and expression of three galactinol synthase genes that confer stress tolerance in Salvia miltiorrhiza. J Plant Physiol 169:1838–1848. doi:10.1016/j.jplph.2012.07.015
Weber H, Heim U, Golombek S, Borisjuk L, Wobus U (1998) Assimilate uptake and the regulation of seed development. Seed Sci Res 8:331–345. doi:10.1017/S0960258500004268
Weber H, Borisjuk L, Wobus U (2005) Molecular physiology of legume seed development. Annu Rev Plant Biol 56:253–279. doi:10.1146/annurev.arplant.56.032604.144201
Yoshida M, Abe J, Moriyama M, Kuwabara T (1998) Carbohydrate levels among winter wheat cultivars varying in freezing tolerance and snow mold resistance during autumn and winter. Physiol Plant 103:8–16. doi:10.1034/j.1399-3054.1998.1030102.x
Zalewski K, Lahuta LB, Horbowicz M (2001) The effect of soil drought on the composition of carbohydrates in yellow lupin seeds and triticale kernels. Acta Physiol Plant 23(1):73–78. doi:10.1007/s11738-001-0025-x
Zhang J, Xu Y, Chen W, Dell B, Vergauwen R, Biddulph B, Khan N, Luo H, Appels R, Van den Ende W (2015) A wheat 1FEH w3 variant underlies enzyme activity for stem WSC remobilization to grain under drought. New Phytol 205(1):293–305. doi:10.1111/nph.13030
Zhao T-Y, Martin D, Meeley RB, Downie B (2004a) Expression of the maize GALACTINOL SYNTHASE gene family: (II) Kernel abscission, environmental stress and myo-inositol influences accumulation of transcript in developing seeds and callus cells. Physiol Plant 121:647–655. doi:10.1111/j.1399-3054.2004.00368.x
Zhao T-Y, Thacker R, Corum JW, Snyder JC, Meeley RB, Obendorf RL, Downie B (2004b) Expression of the maize GALACTINOL SYNTHASE gene family: (I) Expression of two different genes during seed development and germination. Physiol Plant 121:634–646. doi:10.1111/j.1399-3054.2004.00367.x
Zhuo C, Wang T, Lu S, Zhao Y, Li X, Guo Z (2013) A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by myo-inositol and confers multiple tolerances to abiotic stresses. Physiol Plant 149:67–78. doi:10.1111/ppl.12019
Zuther E, Büchel K, Hundertmark M, Stitt M, Hincha DK, Heyer AG (2004) The role of raffinose in the cold acclimation response of Arabidopsis thaliana. FEBS Lett 576:169–173. doi:10.1016/j.febslet.2004.09.006
Acknowledgments
Part of this research was supported by the Polish Ministry of Scientific Research and Higher Education grant 2013/09/B/NZ8/03293.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Gniazdowska-Piekarska.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kellmann-Sopyła, W., Lahuta, L.B., Giełwanowska, I. et al. Soluble carbohydrates in developing and mature diaspores of polar Caryophyllaceae and Poaceae. Acta Physiol Plant 37, 118 (2015). https://doi.org/10.1007/s11738-015-1866-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1866-z