Abstract
In higher plants, glutamine synthetase (GS; EC 6.3.1.2) and glutamate dehydrogenase (GDH; EC 1.4.1.2) are the predominant enzymes in nitrogen metabolism. In this study, we cloned both the GS and GDH genes and analyzed their expression levels and variations in their activity in developing and germinating x Triticosecale (cv. Witon) kernels. The developing kernel samples were collected 3, 5, 7, 9, 13, 15, 20, 25, 30, 35, 40 and 45 days after flowering (DAF). The germinating kernel samples were collected after 8, 16, 24, 48 and 72 h of imbibition. There are two GS isoforms that are localized to different compartments: the cytosol (GS1) and the chloroplast (GS2). Five cDNAs encoding GS proteins in triticale plants were obtained using RT-PCR. We cloned the four genes encoding GS1, which we designated TsGS1-1, TsGS1-2, TsGS1-3 and TsGS1-4 and the only gene encoding GS2, which was designated TsGS2-1. We studied the changes in the enzymatic activity and the expression profiles of the GDH, GS1 and GS2 genes in both the developing and germinating seeds of triticale. Based on our results, there is likely cooperation between GDH and GS1 in the synthesis of glutamine and glutamate during the early stages of seed formation and in the scutella of kernels for up to 24 h of imbibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
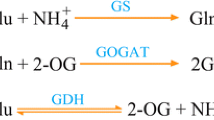
Nitrogen, which is the most important plant nutrient, is a factor which significantly limits the productivity of cereal crops. The NH4 + ion (ammonia) is the only form of nitrogen that can be incorporated into organic compounds by plants (Masclaux-Daubresse et al. 2005). In higher plants, ammonium is mainly assimilated through combined action of glutamine synthetase (GS, EC. 6.3.1.2) and glutamate synthase (GOGAT, EC 1.4.1.14). Alternatively, glutamate dehydrogenase (GDH, EC. 1.4.1.2-4) can also catalyze ammonium incorporation into glutamate by reductive amination of 2-oxoglutarate (Kwinta and Bielawski 1998; Miflin and Habash 2002).
GS catalyzes the ATP-dependent condensation of NH4 + with glutamate to produce glutamine. In plants, GS can exist as distinct isoforms that are classified into groups according to their cellular localization. These isoforms include a cytosolic form, GS1, and a chloroplastic form, GS2 (Bielawski 1993; Miflin and Habash 2002). In general, there is only one gene encoding GS2, with the exception of Medicago truncatula for which two genes were cloned (Seabra et al. 2010); however, a small, multi-gene family encodes for the GS1 proteins (Masclaux-Daubresse et al. 2010). At this time, at least three GS1 genes in rice (Tabuchi et al. 2005), five genes in maize (Martin et al. 2006) and seven genes in wheat (Bernard et al. 2008) have been identified. Studies have shown that both GS isoenzymes are regulated in a developmental manner in the leaves (Habash et al. 2001), and both isoenzymes have different metabolic roles. During the vegetative stage, GS2 assimilates ammonia originating from nitrate reduction and photorespiration (Wallsgrove et al. 1987; Lam et al. 1996). GS1 isoforms are responsible for assimilating the ammonium produced by the reduction of nitrate in the root, and are responsible for synthesizing glutamine for transporting nitrogen between different organs. During leaf senescence, cytosolic GS provides a key function in the assimilation and recycling of ammonia generated from various catabolic processes (Bernard et al. 2008). This role is important after anthesis, during seed development and filling in cereals when nitrogen is remobilized to the reproductive sinks. However, thus far, only two studies have firmly assigned cytosolic GS genes to leaf-related remobilization of assimilate to the seed (i.e., OsGln1;1 in rice (Tabuchi et al. 2005) and Gln1-3, Gln1-4 in maize (Martin et al. 2006).
GDH is found in all plant tissues but its metabolic role is still debated. In vitro, the enzyme catalyzes the reversible amination/deamination reaction between 2-oxoglutarate and glutamate (Kwinta and Bielawski 1998). However, in vivo the reversibility of the reaction catalyzed by GDH is disputed. There is evidence that GDH may operate in the catabolic direction, to fuel the TCA cycle under conditions of carbon limitation (Limami et al. 2008; Miyashita and Good 2008). GDH may also function in the direction of glutamate biosynthesis, under conditions of carbon and ammonium excess (Abiko et al. 2010; Lehmann et al. 2010). GDH is responsible for maintaining the balance between the metabolic pool of carbon and nitrogen compounds during each developmental phase, from seed germination to seed formation (Miflin and Habash 2002). Immunohistochemical studies have determined that the NAD(H)–GDH protein is located mainly in the mitochondria, but occasionally this enzyme is found in the cytoplasm of phloem companion cells (Paczek et al. 2002; Tercé-Laforgue et al. 2004). The native GDH proteins consist of α and β subunits that combine in different ratios to form a hexamer and these combinations create the different GDH isoenzymes (Loulakakis and Roubelakis-Angelakis 1996). In most plant species, GDH is encoded by two distinct nuclear genes and each gene encodes a different subunit α or β (Melo-Oliveira et al. 1996; Restivo 2004). Only one gene coding for the GDH has been cloned from tomato, while the existence of two subunit types has been explained by its post-transcriptional modifications (Purnell et al. 1997). In our previous experiments, we have cloned and characterized one gene encoding GDH in triticale (Grabowska et al. 2011); however, its function in the developing kernels has not been studied in detail.
In the present work, our studies on GS and GDH were performed on developing and germinating seeds from triticale, which is an important feed grain. Triticale grain has a high content of protein and a higher content of lysine compared to wheat. Despite its nutritional advantages, triticale is still poorly characterized at the biochemical and molecular levels, particularly in terms of its nitrogen metabolism. Nitrogen content in the seeds further determines germination efficiency and survival of young seedlings.
This is the first report to present a detailed account on the molecular cloning and characterization of glutamine synthetase genes in triticale. We analyze the expression patterns of these enzymes and assay the activity of two isoforms, TsGS and TsGDH1, in triticale seeds. We investigated the possible roles for GS and GDH during seed development and germination. The results are discussed in the context of our current knowledge of GS and GDH in other plant species.
Materials and methods
Plant material and growth conditions
Triticale x Triticosecale Wittm. cv. Witon were grown in soil in 10-l pots at ten plants per pot in a greenhouse. The soil was supplemented with 2.5 g N/pot in the forms of KNO3 and NH4NO3. The complete nutrient solution contained: 9 % Mg2+, 0.9 % Mn2+, 0.7 % Cu2+, 0.6 % Zn2+, 0.08 % B3+, 0.01 % Mo2+ and 0.005 % Co2+. Plants were vernalized for 6 weeks at 4 °C prior to transplanting in the greenhouse. For the seed development studies, individual spikes were tagged on the day of flowering, and seeds were collected from spikes harvested at different time points: 3, 5, 7, 9, 13, 15, 20, 25, 30, 35, 40 and 45 DAF (days after flowering). The seeds after 45 DAF were the dry grains. For the seed germination studies, dry seeds were surface-sterilized with a solution of 5 % hypochlorite and then germinated in the dark at 22 °C and 100 % relative humidity. Samples were collected after 8, 16, 24, 48, and 72 h of germination. The samples were immediately frozen in liquid nitrogen and stored at −80 °C until use.
Total RNA extraction
Total RNA was extracted from the seeds at 3, 5, 7, 9, 11, 13, 15, 20, 25, 30, 35, 40 and 45 DAF. Total RNA was also collected from the scutella and endosperms of dry seeds that were imbibed for 8, 16, and 24 h, and from the scutella, endosperms, shoots and roots of seeds imbibed for 48 and 72 h. The RNA was isolated using a guanidinium thiocyanate/acidic phenol extraction method (Chomczynski and Sacchi 1987), with an additional initial extraction as described Zdunek-Zastocka (2010). The RNA concentration and purity were determined spectrophotometrically by measuring absorption at 260, 230 and 280 nm. The integrity of the isolated RNA was analyzed on a 1.5 % (w/v) agarose/formaldehyde gel. To eliminate any genomic DNA contamination, the total RNA was treated with RNAse-free DNAase I (Fermentas).
Cloning of the triticale full-length glutamine synthetase cDNA
Four micrograms of total RNA was isolated from a pool of differently developed seeds, scutella, endosperms, shoots and roots imbibed for 48 and 72 h. First-strand cDNA was synthesized using 200 U of Superscript II reverse transcriptase (Invitrogen) and 1 μl biotinylated oligo-dT25 (700 ng ml−1). Conserved domains were identified by aligning the amino acid sequences of several GS1 and GS2 genes identified on the GenBank website. Based on the sequences of the conserved domains, primer pairs (GS1-F/GS1-R and GS2-F/GS2-R) were designed to amplify the core gene fragments (Table 1). PCRs were performed in a PTC-200 Peltier Thermal Cycler (MJ Research) using the following conditions: 2 min at 94 °C, 37 cycles of 30 s at 94 °C, 30 s at 62 °C, 45 s at 72 °C, and a final extension step for 5 min. The PCR was performed in a volume of 50 μl containing 1 μl of cDNA, 0.2 mM of each dNTP, 0.5 μM of each primer, 1× Green GoTaq® Flexi Buffer, 5 mM MgCl2 and 1.25 U of GoTaq® Flexi DNA Polymerase (Promega). The resulting amplicon was cloned into the pGEM-T easy plasmid vector (Promega) and sequenced. Sequencing was performed using the ABI Prism BigDye Terminator Cycle Sequencing Kit on an ABI Prism 3730 DNA analyzer (Applied Biosystems). Trace files were checked and edited using Chromas 1.55 (Technelysium, USA).
The full-length cDNAs were obtained using the GeneRacer Kit (Invitrogen). The RACE-ready, first-strand cDNAs used as the templates for 5′ RACE and 3′ RACE were prepared from total RNA that was isolated from a pool of differently developed seeds, scutella, endosperms, shoots and roots of seeds imbibed for 48 and 72 h. For the 5′ RACE and 3′ RACE reactions, we used either random primers or a GeneRacer OligodT Primer, respectively, according to the manufacturer’s instructions. First-strand synthesis was performed using SuperScript III Reverse Transcriptase (Invitrogen), according to the manufacturer’s instructions. The gene-specific primers used for RACE were designed from the aforementioned, partial TsGS1 and TsGS2 cDNA sequences. In Table 1, only primers used for the amplification of the longest 5′ and 3′ ends are displayed.
The PCR amplification of the 5′ ends of TsGS2-1, TsGS1-1, TsGS1-2, TsGS1-3 and TsGs1-4 was performed using the GeneRacer 5′ primer and gene-specific primers (GSP): rGS2/1-R, rGS1/1-R, rGS1/2-R, rGS1/3-R, rGS1/4-R, respectively, using the following conditions: 2 min at 94 °C, 35 cycles of 30 s at 94 °C, 30 s at X °C (see appropriate temperatures for each primer in Table 1), 1 min at 68 °C, and a final extension for 10 min at 68 . The PCR was performed in a volume of 50 μl containing 1 μl of cDNA template, 0.2 mM of each dNTP, 0.2 μM of GSP, 0.6 μM of GeneRacer 5′ Primer, 1× high fidelity PCR buffer, 5 mM MgCl2 and 2.5 U of Platinum® Taq DNA Polymerase High Fidelity (Invitrogen). The PCR amplifications of the 3′ ends of TsGS2-1, TsGS1-1, TsGS1-2, TsGS1-3 and TsGs1-4 were performed using the GeneRacer 3′ primer and GSP: rGS2/1-F, rGS1/1-F, rGS1/2-F, rGS1/3-F and rGS1/4-F, respectively. The PCR conditions and the reaction mix were the same as described above. Amplification products were cloned into the pCR®4Blunt-TOPO® vector (Invitrogen) and sequenced.
Expression analysis by semi-quantitative RT-PCR
Semi-quantitative RT-PCR analysis was performed using the One-Step RT-PCR Kit (Novagen). The oligonucleotide primer sequences for TsGS1-3, TsGS2-1, and TsGDH1, and the annealing temperatures used for each individual RT-PCR, are displayed in Table 2. The RT-PCRs were performed using the following conditions: 30 min at 60 °C, 2 min at 94 °C, 31 cycles (for TsGS1-3, TsGS2-1 and TsGDH1) and 10 cycles (for rRNA) of 30 s at 94 °C, 30 s at 56 or 60 °C, 30 s at 72 °C and a final extension step for 5 min at 72 °C. The PCR was performed in a total volume of 50 μl containing 100 ng of RNA, 0.2 mM of each dNTP, 0.5 μM of each primer, 1× Reaction Buffer, 5 mM Mn(OAc)2, 15 units of RNase Inhibitor and 5 U Tth Polymerase. The subsequent RT-PCR-generated fragments were cloned into the pGEM-T Easy vector (Promega) and sequenced. To ensure that equal amounts of RNA template were added to each RT-PCR, amplification of the 18S rRNA gene was performed as indicated in Table 2. The amplified products were analyzed by electrophoresis on 1.5 % agarose gels containing ethidium bromide. Densitometry analyses were conducted using the program Image J, version 1.44 (http://www.rsb.info.nih.gov/ij).
Enzyme assays and protein analysis
Glutamate dehydrogenase (GDH) was extracted by homogenizing 0.2 g of developing seeds (3, 5, 7, 9, 13, 15, 20, 25, 30, 35, 40 and 45 DAF) in 1 ml of extraction buffer containing 100 mM Tris–HCl (pH 7.8), 5 mM 2-mercaptoethanol and 20 μM PMSF. An Ultra-Turrax T25 (IKA) homogenizer was used for two, 60 s cycles at 20,000 rpm. The homogenates were centrifuged for 30 min at 10,000×g at 4 °C and the supernatant was used as the enzyme extract. Supernatants were collected and kept on ice for GDH assays and protein concentration analysis. All assays were performed at 30 °C. GDH activity was determined in both the aminating (NADH–GDH) and deaminating (NAD+–GDH) directions by measuring the absorption change at 340 nm (Barash et al. 1973). The standard amination reaction mixture contained 100 mM Tris–HCl (pH 8.3), 200 mM NH4Cl, 0.28 mM NADH, 32 mM 2-oxoglutarate, 0.15 ml of enzyme extract and deionized water, which were added to a final volume of 1.5 ml. The standard deamination reaction mixture contained 100 mM Tris–HCl (pH 9.2), 200 mM l-glutamate, 0.25 mM NAD+, 0.2 ml of enzyme extract and deionized water, which were added to a final volume of 1.5 ml. One unit of GDH activity was defined as the oxidation or reduction of 1 μmol of coenzyme (NADH or NAD+) per min at 30 °C g−1 of dry weight (DW).
Glutamine synthetase (GS) was extracted by homogenizing 0.2 g of developing seeds (3, 5, 7, 9, 13, 15, 20, 25, 30, 35, 40 and 45 DAF), scutella and endosperms of seeds imbibed for 8, 16, 24 h or from scutella, endosperms, shoots and roots of seeds imbibed for 48 and 72 h. Homogenization occurred in a 1 ml extraction buffer containing 100 mM Tris–HCl (pH 7.9), 1 mM EDTA, 20 mM MgSO4, and 1 mM 2-mercaptoethanol. An Ultra-Turrax T25 (IKA) was used for 45 s at 20,000 rpm. The homogenates were centrifuged for 20 min at 10,000×g at 4 °C. The supernatant was used as the enzyme extract. Supernatants were collected and kept on ice for the GS and protein concentration assays. GS activity was measured using the synthetase assay based on the method described by O’Neal and Joy (1973) and optimized for triticale. Two hundred and fifty microliters of enzyme extract was added to 200 μl of assay mix that contained 265 mM glutamate and 60 mM hydroxylamine. The reaction began by the addition of 50 μl of 80 mM ATP and incubated at 30 °C for 30 min. The reaction was stopped by adding 125 μl of 0.5 M trichloroacetic acid. The samples were incubated at 20 °C, followed by centrifugation at 10,000×g for 10 min. Five hundred microliters of supernatant was added to 250 μl of 0.5 M ferric chloride and incubated for 15 min at 20 °C. The controls contained all of the reaction components except for the ATP. The formation of γ-glutamyl hydroxamate (GHM) was determined by measuring the absorbance at 540 nm using a spectrophotometer. The GS activity was expressed as the μmol of GHM per min at 30 °C per DW.
The protein concentrations of the tissue extracts were determined with the Bradford method (Bradford 1976) using bovine serum albumin to create the standard curve. Each measurement was repeated three times, and the results are reported as the mean of two independent biological experiments.
Polyacrylamide gel electrophoresis
Discontinuous native-PAGE was performed as described by Ornstein (1964) and Davis (1964) and according to the manufacturer’s protocol (Mini-Protean Tetra Cell, Bio-Rad). A 4 % stacking gel and a 7.5 % separating gel were used. Equal amounts (50 μg) of soluble protein extracted from the developing seeds were added to each lane. Electrophoresis was performed at 120 V for 1 h at 4 °C in a Tris–glycine buffer system.
After electrophoresis, the protein bands containing GDH activity (deamination) were visualized by incubation in 100 mM Tris–HC1 (pH 9.2) with 100 mM l-glutamate, 0.75 mM NAD, 0.4 mM nitroblue tetrazolium and 0.26 mM phenazine methosulfate for 15–60 min at 37 °C (Hartmann et al. 1973). As a control, an identical gel was incubated in the GDH stain solution minus the glutamate.
Bioinformatics analysis
Sequences were verified by a database search of the National Center for Biotechnology Information server using the BLAST algorithm (http://www.ncbi.nlm.nih.gov). Sequencing dates from this article have been deposited on GenBank. The deduction of the amino acid sequences and calculation of the theoretical molecular masses and pIs were performed using ExPASy (http://www.expasy.ch/tools/). Multiple alignment of the amino acids sequences were generated using the program CLUSTALW (Thompson et al. 1997). Phylogenetic analysis was performed using the neighbor-joining (NJ) method, as implemented in the PhyML program (http://www.phylogeny.fr/version2_cgi/index.cgi) (Dereeper et al. 2010) The bootstrap value was generated using 100 non-parametric replicates. The subcellular localization of the deduced protein was predicted using TargetP (Emanuelsson et al. 2007).
Statistical analysis
The measurements of enzyme activities are presented as the mean ± SD (standard deviation) for three independent experiments, with two replications each. Statistical significance was determined using the Student’s t test. The result was considered to be significant if the P value was less than 0.05 (MS Excel).
Results
Cloning of full-length TsGS cDNAs and sequence analysis of the TsGS and TsGDH1 genes
Using RT-PCR, five TsGS genes were isolated and designated as x Triticosecale cytosolic glutamine synthetase (TsGS1-1, TsGS1-2, TsGS1-3, TsGS1-4; GenBank accession numbers: JN188397, JN188396, JN188395, JN188394, respectively) and plastid glutamine synthetase (TsGS2-1, GeneBank accession number: JN188398). The PCR amplification of the core fragments of the GS1 and GS2 cDNAs was performed using two primer pairs: GS1-F/GS1-R and GS2-F/GS2-R (Table 1). The primers were subsequently used to amplify the 702 and 747 bp fragments of GS1 and GS2, respectively. Ten additional primers (Table 1) were designed based on the confirmed sequences and used to isolate the 5′ and 3′ ends of the sequences.
The full-length TsGS1-1 cDNA is 1,453 bp and contains a 1,071 bp open reading frame (ORF), a 32 bp 5′ untranslated region (5′ UTR) and a 350 bp 3′ UTR. TsGS1-1 encodes a protein containing 356 amino acids with a calculated molecular mass of 39.11 kDa and an isoelectric point of 5.53. The full-length TsGS1-2 cDNA is 1,295 bp and contains a 1,071 bp ORF, a 49 bp 5′ UTR and a 175 bp 3′ UTR. TsGS1-2 encodes a protein containing 356 amino acids with calculated molecular mass of 39.20 kDa and isoelectric point of 5.41. The full-length TsGS1-3 cDNA is 1,440 bp and contains a 1,089 bp ORF, a 134 bp 5′ UTR and a 217 bp 3′ UTR. TsGS1-3 encodes a protein containing 362 amino acids with a calculated molecular mass of 39.50 kDa and an isoelectric point of 5.66. The full-length TsGS1-4 cDNA is 1,142 bp and contains a 1,065 bp ORF and a 77 bp 5′ UTR. TsGS1-4 encodes protein of 354 amino acids with a calculated molecular mass of 38.72 kDa and an isoelectric point of 5.35. Sequence analyses indicated that all TsGS1 proteins contain two conserved domains. The first domain includes an N-terminal beta-Grasp domain from Tyr-23, Glu-17, Gly-17 or Asp-17 to Pro-97 for TsGS1-1, TsGS1-2, TsGS1-3 and TsGS1-4, respectively. The second domain is the C-terminal catalytic domain from residue Pro-103 to Thr-350 (Fig. 1).
The full-length TsGS2-1 cDNA is 1,687 bp long and contains a 1,284 bp open reading frame, a 147 bp 5′ UTR and a 256 bp 3′ UTR. TsGS2-1 encodes a protein of 427 amino acids with a calculated molecular mass of 46.70 kDa and an isoelectric point of 5.75. Hits analyses indicated that TsGS2-1 contains two conserved regions. These domains include an N-terminal beta-Grasp domain from Asp-72 to Pro-152 and a C-terminal catalytic domain from residue Pro-158 to Thr-405. TsGS2-1 had the structure of the plant plastid GS isoform (Fig. 1). The TargetP program predicted that TsGS2-1 contains a chloroplast target peptide (cTP) of 47 residues on its N-terminus (score 0.752, TargetP) (Fig. 1). With the cTP sequence removed, the calculated molecular mass of the mature TsGS2-1 protein is 42.70 kDa and has an isoelectric point of 5.02.
The previously cloned full-length TsGDH1 gene (Grabowska et al. 2011) encoded 411 amino acids. The deduced amino amid sequence TsGDH1 contained two features present in all GDH proteins. First, a Glu/2-OG binding site from Ile-96 to Pro-109, and second, an NAD(H)-binding site from Phe-209 to Asp-237 (Britton et al. 1992). Additionally, TsGDH1 contains a mitochondrial targeting peptide (mTP) on its N-terminus (score 0.323, TargetP).
Homology and phylogenetic analysis of plant glutamine synthetase
The sequence alignment analyses revealed that TsGS1s are highly homologous at both the nucleotide and the deduced amino acid levels. TsGS1-1 shares a 97 % identity with TsGS1-2, and 79 and 81 % identities with TsGS1-3 and TsGS1-4, respectively. The TsGS1-2 sequence is 79 % identical to TsGS1-3 and 82 % identical to TsGS1-4. TsGS1-3 shares a 79 % identity with TsGS1-4. A similar identity percentage between TsGS1 sequences was observed at the amino acid level. For TsGS2-1, sequence identity at the nucleotide and the deduced amino acid levels with all TsGS1s was approximately 60 %. All TsGSs genes are highly homologous to wheat genes. TsGS1-1 shares a 99.6 % identity with GS1a (DQ124209), TsGS1-2 shares a 99.9 % identity with GS1b (DQ124210), TsGS1-3 shares a 99.1 % identity with GSe2 (AY491971), TsGS1-4 and TsGS2-1 shares a 100 % identity with GSr2 (AY491969) and GS2c (DQ124214), respectively.
To investigate the evolutionary relationships between the TsGSs and GSs from other plant species, a phylogenetic tree was constructed based on the deduced amino acid sequences of the GS genes. The phylogenetic analysis revealed that all plant GSs are closely related and can be grouped into two clusters (Fig. 2). All cloned TsGSs genes included orthologous genes from the major crops species: wheat, maize and rice. Triticale is a hybrid of wheat and rye. The presence of ten GS genes in wheat may suggest the occurrence of additional genes in triticale. The phylogenetic analysis indicated that all members of GS1 and GS2 were classified into one group, respectively. TsGS2-1 clustered with the GS2s from M. truncatula (AAO37651, ADM45299, ADM45300), Triticum aestivum (AAZ30060, AAZ30061, AAZ30062), Oryza sativa (P14655), Zea mays (P25462) and Secale cereale (AFB698778).
Phylogenetic analysis of glutamine synthetase from different plant species. The number above each branch represents the bootstrap support for 100 replicates. The analysis was based on the deduced amino acid sequences of the GS genes. The names of the genes are given in brackets. The accession numbers ADM45299, ADM45300 and AAO37651 correspond to the Medicago truncatula (Mt) proteins; AAZ30060, AAZ30061, AAZ30062, AAZ30057, AAZ30058, AAZ30059, AAR84347, AAR84348, AAR84349 and AAR84350 correspond to the Triticum aestivum (Ta) proteins; ABF698778, ABF698779, ABF69880 correspond to the Secale cereale (Sc) proteins, P14656, P14654, Q4W8D0 and P14655 correspond to the Oryza sativa proteins (Os); P38559, P38560, P38561, P38562, P38563 and P25462 correspond to the Zea mays (Zm) proteins; ABW89466 corresponds to the Gossypium hirsutum (Gh) protein; AEM42903, AEM42902, AEM42901, AEM42900 and AEM42904 correspond to the x Triticosecale (Ts) proteins
The GS1 cluster is subdivided into three groups. The first group contained one of three rice GS1 genes (P14656), two of the five maize GS1 genes (P38561 and P38562), three of the seven wheat GS1 genes (AAZ30057, AAZ30058 and AAZ30059), one of the two rye GS1 genes (AFB69879) and two of the four triticale GS1 genes (TsGS1-1 and TsGS1-2). Other GS1 members clustered in the second group with one of the five maize GS1 genes (P38560), one of the three rice GS1 genes (Q4W8D0), two of the seven wheat GS1 genes (AAR84350 and AAR84349), one of the four triticale GS1 genes (TsGS1-3) and one cotton GS1 gene (ABW89466). The third class contained two of the five GS1 genes found in both maize and wheat (maize: P38563, P38559; wheat: AAR84347, AAR84348), one of the two rye GS1 genes (AFB69880), one of the three rice GS1 genes (P14654) and one of the four triticale genes (TsGS1-4) (Fig. 2).
Expression analysis by semi-quantitative RT-PCR
Expression of the TsGDH1, TsGS1-3 and TsGS2-1 genes were investigated using semi-quantitative RT-PCR of the total RNA isolated from seeds during different developmental stages (Fig. 3). In addition, expression analysis was performed on the TsGS1-3 and TsGS2-1 genes in the scutella and endosperms of dry seeds, or seeds imbibed for 8, 16 or 24 h. Expression analysis was also performed on these two genes in the scutella, endosperms, shoots and roots of seeds imbibed for 48 and 72 h (Fig. 4). The expression of TsGS1-1, TsGS1-2 and TsGS1-4 genes displayed low transcript levels in the triticale seeds (data not shown). Based on these results and the phylogenetic analysis (Fig. 2), we chose to analyze the expression of the cytoplasmic TsGS1-3 gene.
Expression of the TsGDH1, TsGS1-3 and TsGS2-1 genes in the developing triticale seeds. Quantitative, one-step, RT-PCR analyses were performed using 100 ng of total RNA. Amplification of the 18S rRNA gene was used to ensure that equal amounts of the template were added to each RT-PCR. Each figure represents one of at least three independent experiments that all yielded similar results. The relative gene expression intensities were quantified using NIH Image Software (Version 1.44). DAF days after flowering
Expression of the TsGS1-3 and TsGS2-1 genes in different seed compartments, and their expression patterns during germination. E-0 endosperms of dry seeds; S-0 scutella from dry seeds; E-8 endosperms from seeds imbibed for 8 h; S-8 scutella from seeds imbibed for 8 h; E-16 endosperms from seeds imbibed for 16 h; S-16 scutella from seeds imbibed for 16 h; E-24 endosperms from seeds imbibed for 24 h; S-24 scutella from seeds imbibed for 24 h; E-48 endosperms from seeds imbibed for 48 h; S-48 scutella from seeds imbibed for 24 h; R-48 roots from seeds imbibed for 48 h; Sh-48 shoots from seeds imbibed for 48 h; E-72 endosperms from seeds imbibed for 72 h; S-72 scutella from seeds imbibed for 72 h; R-72 roots from seeds imbibed for 72 h; Sh-72 shoots from seeds imbibed for 72 h. Quantitative, one-step, RT-PCR analyses were performed using 100 ng of total RNA. Amplification of the 18S rRNA gene was used to ensure that equal amounts of the template were added to each RT-PCR. Each figure represents one of at least three independent experiments that all yielded similar results. The relative gene expression intensities were quantified using NIH Image Software (Version 1.44)
The TsGDH1 mRNA was detected at all stages of seed development. The highest level (100 % relative intensity) in the seeds was observed at 15 DAF. The TsGDH1 transcripts levels were high at 3 DAF and then gradually declined until 9 DAF. High levels were again observed at 13, 15 and 20 DAF. This was followed by a gradual decrease in transcript levels until 30 DAF, and subsequently, a further increase in transcript levels during the final stages of seed formation. The level of TsGS1-3 transcript gradually increased during the early stages of kernel development, reaching its maximum at 13 DAF (100 % relative intensity), and then gradually declined to very low levels at 45 DAF. The transcript level of TsGS2-1 was at the highest at 7 DAF (100 % relative intensity), and then gradually declined to very low levels from 25 to 45 DAF (Fig. 3).
The expression levels of the TsGS1-3 and TsGS2-1 genes were also studied in dry and germinating triticale seeds imbibed for 8, 16, 24, 48 and 72 h. The transcripts of both genes were detected in all examined tissues. The highest expression levels were observed in the scutella of seeds imbibed for 24 h (100 % relative intensity), and the shoots of seeds imbibed for 72 h, for TsGS1-3 and TsGS2-1, respectively. The transcript level of TsGS1-3 was also high in the scutella of seeds imbibed for 8 and 16 h, and in the endosperms and the roots of seeds imbibed for 72 h. The lowest level of the TsGS1-3 transcript was observed in the endosperms of dry seeds. TsGS2-1 transcript levels were high in the shoots of seeds imbibed for 48 h, and in the scutella of seeds imbibed for 72 h (Fig. 4).
Glutamate dehydrogenase and glutamine synthetase activity
The process of kernel development is accompanied by the synthesis of many nitrogen storage substances, including amino acids and proteins. The development of triticale seeds can be separated into three phases: seed formation, filled grains and maturation and desiccation.
During each stage of kernel development studied, both aminating (NADH–GDH) and deaminating activity (NAD+–GDH) were determined. During the initial phase of kernel development, the assayed activity levels of the NADH-dependent GDH were much higher than the deaminating activity (NAD+–GDH). Particular attention should be paid to the very high aminating activity of the enzyme, which was equal to 8.53 μmol of oxidized coenzyme (ox. coenz.) min−1 g−1 DW at 3 DAF. This activity rapidly dropped during the next few days (i.e., at 5 and 7 DAF) to reach the maximum value of 8.56 μmol ox. coenz. min−1 g−1 DW at 11 DAF (Fig. 5).
Similar trends were observed in the changes in deaminating activity of the NAD+-dependent GDH enzyme. The samples collected on consecutive days (i.e., 13–45 DAF) displayed a steady decline in their NADH-GDH activity. The lowest aminating activity of the enzyme (0.87 μmol ox.coenz. min−1 g−1 DW) was assayed in the seeds on 45 DAF. From 25 to 30 DAF, an increased activity of NAD+–GDH, then gradual decrease was observed. In the dry grain, the GDH deaminating activity exceeded the aminating activity. During kernel formation (3–20 DAF), a significant advantage of the GDH aminating activity over its deaminating activity was determined, and the ratio (aminating activity/deaminating activity) ranged from 8 to 5.3. During the grain maturation phase, from 25 to 45 DAF, the ratio of NADH–GDH to NAD+–GDH gradually declined. Indeed, at 45 DAF the ratio was 0.77 (Fig. 5).
Preparations obtained from the developing kernels (3–45 DAF) were also analyzed electrophoretically under non-denaturing conditions. After staining the gels for NAD+–GDH activity, a single band of enzyme activity was visualized (Fig. 6).
For glutamine synthetase, a high level of activity in the developing seeds was observed as early as 3 DAF. On subsequent days of kernel formation, the activity increased and reached its maximum at 15 DAF (7.91 μmol GHM min−1 g−1 DW). At 20 and 25 DAF, the activity of the enzyme declined sharply relative to the maximum value (1.6- and 4.3-fold, respectively). In later stages of seed development, the GS activity gradually decreased and was only 0.19 μmol GHM min−1 g−1 DW at 45 DAF (Fig. 7).
Glutamine synthetase activity was measured in the scutella and endosperms of dry seeds, as well as in seeds imbibed for 8, 16 and 24 h. GS activity was also measured in the scutella, endosperms, shoots and roots of seeds imbibed for 48 and 72 h. The lowest level of GS activity (0.15 μmol GHM min−1 g−1 DW) was observed in the endosperm of the dry seeds. During the next imbibition time points, the GS activity level in the endosperm gradually increased and reached its maximum level at 72 h (1.11 μmol GHM min−1 g−1 DW). In the scutella of dry seeds, the GS activity level was six times higher relative to the activity in the endosperm of the dry seeds (0.90 μmol GHM min−1 g−1 DW). During imbibition, the GS activity level in the scutellum gradually increased to 3.19 μmol GHM min−1 g−1 DW at 72 h. The levels of the GS activity in the shoot after 48 and 72 h of imbibition were 1.89 and 4.44, respectively. After 48 and 72 h of imbibition in the root, the GS activity levels were 1.77 and 2.54 μmol GHM min−1 g−1 DW, respectively (Fig. 8).
Discussion
Nitrogen is an essential nutrient that plays a major role in determining the amount of protein stored in cereal grains. The content and quality of protein are major determinants of the nutritional value of cereal grains and grain-derived products. The accumulation of storage proteins in the seed involves N-remobilization from vegetative organs, which is a process involving the GS and GDH enzymes. Although both enzymes have been thoroughly investigated in vegetative plant organs (Masclaux-Daubresse et al. 2005), they have been poorly studied in seeds.
Similar to barley, the development of triticale seeds can be split into three main stages. The initial phase occurs approximately two weeks post-anthesis when the endosperm cellularizes and the organelles proliferate. The second phase is characterized by the rapid synthesis of storage products. The third phase occurs approximately 30 days after anthesis. During this phase, the dry matter accumulation rate decreases and the grains begin to desiccate (Ma and Smith 1992; Goldberg et al. 1994). Maturation, which is the final stage of seed development, is characterized by a dehydration phase that leads to a loss of more than 90 % of water content and a dramatic decrease in metabolic activity (Baskin and Baskin 1998). The induction of germination after seed rehydration induces an increase in both respiration and metabolic activity, thereby allowing the mobilization of N reserves.
This detailed study of the GS gene family in triticale has increased our understanding of the function of specific GS isoenzymes. As expected, the phylogenetic analysis indicated that the TsGS2 gene was located on the same branch as other plant species’ GS2 genes. Three isoforms of wheat GS2 (AAZ300060, AAZ300061 and AAZ300062) were located on the same branch as TsGS2-1. The TsGS2-1 gene also clustered near three M. truncatula genes that are exclusively expressed in developing seeds (i.e., MtGS2b-α (ADM45299), MtGS2b-β (ADM45300) and MtGS2a (AAO37651)) (Seabra et al. 2010). The TsGS1-3 gene, which was used in the expression analysis of the developing and germinating triticale seeds, clustered near three genes (i.e., OsGln1;1 (P14656) in rice and Gln1-3, (P38562), Gln1-4 (P38561) in maize). These three genes have similar physiological functions that are involved in controlling seed development (Tabuchi et al. 2005; Martin et al. 2006).
Two triticale genes, TsGS1-1 and TsGS1-2, were located on the same branch of the phylogenetic tree in close proximity to OsGln1;1, Gln1-3 and Gln1-4. The expression of these two genes displayed low transcript levels in the triticale seeds (data not shown). The phylogenetic analysis demonstrates that the TsGS1-3 gene is closely related to GhGS (ABW89466) and GS1-2 (P38560) from cotton and maize, respectively. A study by Yajun et al. (2008) demonstrated that GhGS may be associated with ovule development. Another gene located on the same branch of the phylogenetic tree as TsGS1-3 is the GS1-2 maize gene (Li et al. 1993). This gene is one of five genes encoding for the cytoplasmic forms of GS and encodes a unique isozyme, GSp1 (Muhitch 1989). GSp1 is strongly expressed in the basal maternal tissues of the developing seed (Rastogi et al. 1998). To date, examination of the tissue distribution of GSp1 has been limited to the endosperm, embryo, pedicle, roots and leaves (Muhitch 1989). Expression of a GS1-2 promoter/GUS heterologous gene resulted in the predicted tissue-specific expression patterns in the seeds. Indeed, the expression pattern of GS1-2 within the kernel is consistent with the GSp1 isozyme’s recognized role in nitrogen metabolism during grain filling (Muhitch 2003). The TsGS1-3 gene is also closely related to GSe1 (AAR84349) and GSe2 (AAR84350) from wheat. The level of GSe mRNA was very low in root, leaf, peduncle and glume (Bernard et al. 2008). In our study a high level of expression of TsG 1-3 gene was observed in the triticale seeds.
To determine the roles of GS and GDH in the developing and germinating seeds of triticale, we conducted studies to elucidate the relationship between the dynamic activity changes of these enzymes and the expression patterns of the GS and GDH genes.
The high activity of GS during the first phase of seed development confirms the key role of this enzyme in the assimilation of ammonia derived from nitrogen remobilization (Lea and Miflin 2003). The aminating activity of GDH was highest during the first phase of kernel development, and this activity level decreased during the later stages of seed development. This result may indicate that GDH also participates in the assimilation of ammonia derived from nitrogen remobilization (Tercé-Laforgue et al. 2003). Similar results were obtained for both maize seeds (Hadzi-Taskovic Sukalovic 1984) and the Malno variety of triticale (Kwinta et al. 1999). Because the presented GS activity profile is the sum of both GS1 and GS2 activities, we conducted expression analyses on the genes encoding the cytoplasmic and the chloroplastic forms to determine the contribution of these isoenzymes to the assimilation of nitrogen during seed development. For TsGS2-1, the highest level of expression was observed at 7 DAF. At this same time point, the expression levels of both TsGS1-3 and TsGDH1 were lower.
In M. truncatula seeds from 3 to 33 DAF, Seabra et al. (2010) observed high transcript levels of one of the genes encoding the chloroplast GS isoenzyme. During the first phase of seed development in triticale, a possible function for the GS2 enzyme is in N-remobilization from vegetative organs to glutamine required for storage protein synthesis. For the TsGDH1 and TsGS1-3 genes, the maximum transcript levels were observed from 11 to 20 DAF, and both genes were simultaneously expressed. In addition, the high levels of TsGDH1 transcript were also observed at 3 DAF. Similarly, Hansen et al. (2009) demonstrated that during the early stages of barley seed development, the expression of genes encoding GS1 was accompanied by the expression of glutamate dehydrogenase genes. While the high transcript level of the genes encoding GS explains the role of this enzyme in nitrogen assimilation (Bernard and Habash 2009), the high level of TsGDH1 may be surprising. However, alternative pathways, such as the reaction catalyzed by glutamate dehydrogenase, may be involved in the assimilation of nitrogen in the seeds. When the expression levels of TsGS1-3 and TsGS2-1 are low, TsGDH1 gene expression was high, thus compensating for the low expression levels of the GS genes in seeds. Similar conclusions were drawn by Tabuchi et al. (2005). This suggests the potential participation of GDH in nitrogen assimilation in rice when OsGS1-1 is missing.
During the third phase of seed development, when the dry matter accumulation rate decreases and grains begin to desiccate, a sharp decline in GS activity was observed in parallel with a decrease in TsGS1-3 and the low expression levels of TsGS2-1. Simultaneously, the high transcript level of TsGDH1 gene and the high deaminating activity (30 and 35 DAF) were observed. However, in dry seeds the deaminating activity was decrease. The high deaminating activity observed during the third phase of seed development (25–45 DAF) probably is part of the metabolic mechanism associated with the startup of protein storage. This is due to the fact that during kernel germination, amino acids, including glutamic acid, are the primary sources of nitrogen available to the growing plants. This correlates with the results of previous studies on the germination of triticale seeds (Grabowska et al. 2011).
Our previous experiments performed on germinating triticale seeds determined that the deaminating reaction catalyzed by GDH is predominant during the first phase of imbibition (Grabowska et al. 2011). The high transcript level of TsGDH1 in the germinating triticale seeds coincides with the high expression levels of TsGS1-3. This may indicate a functional cooperation of these genes’ protein products. The high activity of NAD+–GDH observed during the early stage of triticale germination (Grabowska et al. 2011) is responsible for the degradation of glutamic acid. It may be possible that this is the mechanism used to deliver ammonia to the reaction catalyzed by GS1, and to provide the carbon skeletons for respiration (Aubert et al. 2001).
The germinating triticale seeds displayed substantially higher levels of GS activity in the scutella compared to the endosperms. Within the seed, the scutellum is especially interesting because of its important role in nitrogen mobilization. Also, Marttila et al. (1993) observed an increase in the GS activity in the scutellum during barley seed germination. The transient induction of a seed-specific GS isoenzyme was also observed during the imbibition of sunflower seeds (de la Haba et al. 1992). Up to 48 h of imbibition, a higher level of TsGS1-3 gene expression was observed in both the endosperm and the scutellum compared to the gene encoding the chloroplastic form of GS. This finding suggests that TsGS1-3 is likely involved in the reassimilation of ammonia released from the amino acids that were the result of the proteolysis of storage proteins in the seed. In cotyledons and the embryo axis of M. truncatula, Glevarec et al. (2004) also observed high levels of the transcript encoding the cytoplasmic form during the first phase of germination. In shoots from triticale seedlings, high levels of TsGS2-1 expression, which correlated with the high activity of GS, were observed after 48 and 72 h of imbibition. This finding may suggest that GS2, rather than the cytoplasmic form, has a greater contribution to the total enzyme activity in the shoots of triticale seedlings. In the roots of triticale seedlings, however, the contribution of both GS isoforms to the overall activity of the enzyme appears to be similar, which is apparent from the expression analyses of TsGS1-3 and TsGS2-1.
To the best of our knowledge, the present work demonstrates, for the first time, the enzymatic activity and the expression of the GS1, GS2 and GDH genes in seeds. We discuss the possible roles for these genes/enzymes during the development and germination of triticale seeds. The confirmation of a potential relationship between the TsGSs genes identified in this study and TsGDH1 requires further research, including mutant analyses.
Authors contribution
Agnieszka Grabowska and Joanna Kwinta designed research, performed experiments analyzed data, and wrote the paper. Agnieszka Grabowska PhD cloned all genes, performed semi-quantified gene expression, and conducted all bioinformatics analyses. Joanna Kwinta PhD performed enzymes assays, protein analysis, and polyacrylamide gel electrophoresis. Wiesław Bielawski supervised the project and gave technical support. All authors discussed the results and implications and commented on the manuscript at all stages. All authors take full responsibility for the content of the paper.
References
Abiko T, Wakayama M, Kawakami A, Obara M, Kisaka H, Miwa T et al (2010) Changes in nitrogen assimilation, metabolism, and growth in transgenic rice plants expressing a fungal NADP(H)-dependent glutamate dehydrogenase (gdhA). Planta 232:299–311. doi:10.1007/s00425-010-1172-3
Aubert S, Bligny R, Douce R, Gout E, Ratcliffe RG, Roberts JK (2001) Contribution of glutamate dehydrogenase to mitochondrial glutamate metabolism studied by 13C and 31P nuclear magnetic resonance. J Exp Bot 52:37–45. doi:10.1093/jexbot/52.354.37
Barash I, Sadon T, Mor H (1973) Induction of a specific isoenzyme of glutamate dehydrogenase by ammonia in oat leaves. Nat New Biol 244:150–152. doi:10.1038/newbio244150a0
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego
Bernard SM, Habash DZ (2009) The importance of cytosolic glutamine synthetase in nitrogen assimilation and recyling. New Phytol 182:608–620. doi:10.1111/j.1469-8137.2009.02823.x
Bernard SM, Blom Møller AL, Dionisio G, Jahn TP et al (2008) Gene expression and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Mol Biol 67:89–105. doi:10.1007/s11103-008-9303-y
Bielawski W (1993) Distribution of glutamine synthetase isoforms in triticale seedling leaves. Acta Physiol Plant 15:211–218
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Britton KL, Baker PJ, Rice DW, Stillman TJ (1992) Structural relationship between the hexameric and tetrameric family of glutamate dehydrogenases. Eur J Biochem 209:851–859. doi:10.1111/j.1432-1033.1992.tb17357.x
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159
Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann New York Acad Sci 121:404–427. doi:10.1111/j.1749-6632.1964.tb14213.x
de la Haba P, Cabello P, Maldonado JM (1992) Glutamine-synthetase isoforms appearing in sunflower cotyledons during germination: effects of light and nitrate. Planta 186:577–581. doi:10.1007/BF00198038
Dereeper A, Audic S, Claverie JM, Blanc G (2010) BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol 10:8. doi:10.1186/1471-2148-10-8
Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using Target P, Signal P and related tools. Nat Protoc 4:953–971. doi:10.1038/nprot.2007.131
Glevarec G, Bouton S, Jaspard E, Riou M-T, Cliguet B, Suzuki A, Limami AM (2004) Respective roles of the glutamine synthetase/glutamate synthase cycle and glutamate dehydrogenase inammonium and amino acid metabolism during germination and post-germinative growth in the model legume Medicago truncatula. Planta 219:286–297. doi:10.1007/s00425-004-1214-9
Goldberg RB, de Paiva G, Yadegari R (1994) Plant embryogenesis: zygote to seed. Science 266:605–614. doi:10.1126/science.266.5185.605
Grabowska A, Nowicki M, Kwinta J (2011) Glutamate dehydrogenase of the germinating triticale seeds: gene expression, activity distribution and kinetic characteristics. Acta Physiol Plant 33:1981–1990. doi:10.1007/s11738-011-0801-1
Habash DZ, Massiah AJ, Rong HL, Wallsgrove RM, Leigh RA (2001) The role of cytosolic glutamine synthetase in wheat. Ann Appl Biol 138:83–89. doi:10.1111/j.1744-7348.2001.tb00087.x
Hadzi-Taskovic Sukalovic V (1984) Activity of nitrogen metabolism enzymes in the process of kernel development in different maize genotypes. FEBS Lett 171:59–62
Hansen M, Friis C, Bowra S, Holm P, Vincze E (2009) A pathway-specific microarray analysis highlights the complex and co-ordinated transcriptional networks of the developing grain of field-grown barley. J Exp Bot 60:153–167. doi:10.1093/jxb/ern270
Hartmann T, Nagel M, Ilert HJ (1973) Organ-specific multiple forms of glutamate dehydrogenase in Medicago sativa. Planta 111:119–128
Kwinta J, Bielawski W (1998) Glutamate dehydrogenase in higher plant. Acta Physiol Plant 20:453–463. doi:10.1007/s11738-998-0033-1
Kwinta J, Bartoszewicz K, Bielawski W (1999) Glutamate dehydrogenase and glutamate synthetase activities during development of Triticale grains. Acta Physiol Plant 21:271–275. doi:10.1007/s11738-999-0042-8
Lam HM, Coschigano KT, Oliveira IC, Melo-Oliveira R, Coruzzi GM (1996) The molecular genetics of nitrogen assimilation into amino acids in higher plants. Ann Rev Plant Physiol Plant Mol Biol 47:569–593. doi:10.1146/annurev.arplant.47.1.569
Lea PJ, Miflin BJ (2003) Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol Biochem 41:555–564. doi:10.1016/S0981-9428(03)00060-3
Lehmann T, Skrok A, Ratajczak L (2010) Stress-induced changes in glutamate dehydrogenase activity imply its role in adaptation to C and N metabolism in lupine embryos. Physiol Plant 138:35–47. doi:10.1111/j.1399-3054.2009.01294.x
Li M, Villemur R, Hussey PJ, Silflow CD, Gantt S, Snustad PD (1993) Differential expression of six glutamine synthetase genes in Zea mays. Plant Mol Biol 23:401–407. doi:10.1007/BF00029015
Limami AM, Glevarec G, Ricoult C, Cliquet JB, Planchet E (2008) Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedlings under hypoxic stress. J Exp Bot 59:2325–2335. doi:10.1093/jxb/ern102
Loulakakis KA, Roubelakis-Angelakis KA (1996) The seven NAD(H)-glutamate dehydrogenase isoenzymes exhibit similar anabolic and catabolic activities. Plant Physiol 96:29–35. doi:10.1111/j.1399-3054.1996.tb00179.x
Ma BL, Smith DL (1992) Apical development of spring barley under field conditions in northeastern North America. Crop Sci 32:144–149
Martin A, Lee J, Kichey T et al (2006) Two cytosolic glutamine synthetase isoforms of maize (Zea mays L.) are specifically involved in the control of grain production. Plant Cell 18:3252–3274. doi:10.1105/tpc.106.042689
Marttila S, Saarelainen R, Porali I, Mikkonen A (1993) Glutamine synthetase isozymes in germinating barley seeds. Physiol Plant 88:612–620
Masclaux-Daubresse C, Carrayol E, Valadier M-H (2005) The two nitrogen mobilisation- and senescence-associated GS1 and GDH genes are controlled by C and N metabolites. Planta 221:580–588. doi:10.1104/pp.105.071910
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157. doi:10.1093/aob/mcq028
Melo-Oliveira R, Oliveira IC, Coruzzi GM (1996) Arabidopsis mutant analysis and gene regulation define a non-redundant role for glutamate dehydrogenase in nitrogen assimilation. Proc Natl Acad Sci USA 93:4718–4723
Miflin BJ, Habash DZ (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53:979–987. doi:10.1093/jexbot/53.370.979
Miyashita Y, Good AG (2008) NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J Exp Bot 59:667–680. doi:10.1093/jxb/erm340
Muhitch MJ (1989) Purification and characterization of two forms of glutamine synthetase from the pedicel region of maize (Zea mays L.) kernels. Plant Physiol 91:868–875
Muhitch MJ (2003) Distribution of the glutamine synthetase isozyme GSp1 in maize (Zea mays). J Plant Physiol 160:601–605. doi:org/10.1078/0176-1617-01046
O’Neal D, Joy KW (1973) Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys 159:113–122
Ornstein L (1964) Disc electrophoresis. I Background and theory. Ann New York Acad Sci 121:321–349
Paczek V, Dubois F, Sangwan R, Morot-Gaudry JF, Roubelakis-Angelakis KA, Hirel B (2002) Cellular and subcellular localisation of glutamine synthetase and glutamate dehydrogenase in grapes gives new insights on the regulation of C and N metabolism. Planta 216:245–254. doi:10.1007/s00425-002-0854-x
Purnell MP, Stewart GR, Botella JR (1997) Cloning and characterization of a glutamate dehydrogenase cDNA from tomato (Lycopersicum esculentum L.). Gene 186:249–254. doi:org/10.1016/S0378-1119(96)00716-0
Rastogi R, Chourey PS, Muhitch MJ (1998) The maize glutamine synthetase GS1-2 gene is preferentially expressed in kernel pedicels and is developmentally regulated. Plant Cell Physiol 39:443–446
Restivo FM (2004) Molecular cloning of glutamate dehydrogenase genes of Nicotiana plumbagifolia: structure analysis and regulation of their expression by physiological and stress conditions. Plant Sci 166:971–982. doi:org/10.1016/j.plantsci.2003.12.011
Seabra AR, Vieira CP, Cullimore JV, Carvalho HG (2010) Medicago truncatula contains a second gene encoding a plastid located glutamine synthetase exclusively expressed in developing seeds. BMC Plant Biol 10:183. doi:10.1186/1471-2229-10-183
Tabuchi M, Sugiyama K, Ishiyama K, Inoue E, Sato T, Takahashi H, Yamaya T (2005) Severe reduction in growth rate and grain filling of rice mutants lacking OsGS1;1, a cytosolic glutamine synthetase1;1. Plant J 42:641–651. doi:10.1111/j.1365-313X.2005.02406.x
Tercé-Laforgue T, Mäck G, Hirel B (2004) New insights towards the function of glutamate dehydrogenase revealed during source-sink transition of tobacco (Nicotiana tabacum L.) plants grown under different nitrogen regimes. Plant Physiol 120:220–228. doi:10.1111/j.0031-9317.2004.0241.x
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Wallsgrove RM, Turner JC, Hall NP, Kendall AC, Bright SW (1987) Barley mutants lacking chloroplast glutamine synthetase: biochemical and genetic analysis. Plant Physiol 83:155–158. doi:org/10.1104/pp.83.1.155
Yajun H, Wangzhen G, Xinlian S, Tianzhen Z (2008) Molecular cloning and characterization of a cytosolic glutamine synthetase gene, a fiber strength-associated gene in cotton. Planta 228:473–483. doi:10.1007/s00425-008-0751-z
Zdunek-Zastocka E (2010) The activity pattern and gene expression profile of aldehyde oxidase during the development of Pisum sativum seeds. Plant Sci 179:543–548. doi:org/10.1016/j.plantsci.2010.08.005
Acknowledgments
This work was supported, in part, by the Ministry of Science and Higher Education (Grant NN310301134).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klobus.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Grabowska, A., Kwinta, J. & Bielawski, W. Glutamine synthetase and glutamate dehydrogenase in triticale seeds: molecular cloning and genes expression. Acta Physiol Plant 34, 2393–2406 (2012). https://doi.org/10.1007/s11738-012-1085-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1085-9