Abstract
Drought tolerance in barley is highly correlated with the expression of two genes: Hordeum vulgare aleurone 1 (HVA1) and stress-responsive gene 6 (SRG6). Though their role in the mechanism of drought response in barley has been confirmed in transgenic plants, the regulation pathways of these genes’ expression have not been sufficiently studied, especially on the level of whole plants. We used four barley genotypes of different drought tolerance to establish and compare the expression profiles of SRG6 and HVA1 and to associate them with the possible physiological and biochemical signals of water deficit. Both genes studied were expressed to a greater extent in drought tolerant genotypes. The highest level of HVA1 transcript accumulation was observed under conditions where the leaf water potential decreased significantly. In tolerant genotype this signal was partially replaced with abscisic acid (ABA) signal of soil water deficit and the final transcript accumulation was about 14 times lower than in the case of leaf water deficit. In the case of SRG6 the main signal which can triggered the transcript accumulation was ABA but in the case of tolerant genotype the direct effect of leaf water deficit was also observed. Thus, it seems to be possible that in drought tolerant barley genotypes, HVA1 and SRG6, are not only more expressed during drought, but tolerant genotypes may be also more sensitive to various internal signals confirming environmental water deficit. The putative role of hydrogen peroxide as a signal of water deficit in the regulation of both genes expression was not confirmed. The drought-induced expression of both HVA1 and SRG6 was additionally reduced in the light. Because of this powerful complexity, a true understanding of plant response to drought requires further studies integrating gene expression and cell signaling analysis in single organs or tissues with whole plant physiology and long distance signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crop productivity, including that of two-row barley (Hordeum distichon), is lower in most cases than the yielding potential determined by the climate and soil conditions of the cultivated area. Drought is considered to be the main environmental factor causing this phenomenon (Jana and Wilen 2005). Drought tolerance is under complex genetic control, and many genes are involved in plant response to water deficit (Tuberosa and Salvi 2006; Cattivelli et al. 2008). The expression of these genes is controlled by various cellular signals, and the exact function of different signaling pathways is still unclear. An effort is being made to recognize signals of decreased turgor pressure and water loss in single cells (Boudsocq and Laurière 2005). Such signals directly trigger gene expression and induct the biosynthesis of abscisic acid (ABA), which may partially regulate different sets of the genes. At the whole plant level, the drought response is much more complicated. Plants must respond early and fast to soil water deficit and activate protective mechanisms in shoots to minimize the negative effects of drought. Thus, several long distance non-hydraulic signaling mechanisms were identified during drought response (Schachtman and Goodger 2008). In this context, ABA is considered to be the main compound transported in xylem sap to shoots and controlling both their stomatal closure and the expression of genes potentially involved in drought response (Yang et al. 2006; Schachtman and Goodger 2008).
Under drought, a rapid ROS accumulation, including hydrogen peroxide (H2O2), is observed (Bian and Jiang 2009). H2O2 may act as a specific signal regulating gene expression and activity in plants subjected to environmental stresses, including drought (Foyer and Noctor 2005; Schachtman and Goodger 2008; Vandenbroucke et al. 2008). H2O2 could also act as a regulator for stomatal behavior in optimizing water use efficiency under drought (Xu et al. 2010) and be a signal between root and shoot in a stress response (Yang et al. 2006). In drought, when photosynthetic carboxylation is restricted, an imbalance between light energy supply and its utilization was observed. Not only does the oxidative stress increase, but also an additional signal for gene expression connected with the PSII redox state can be induced (Pfannschmidt et al. 2009). Thus, the potential interaction between light and water deficit may be observed in regulation of gene expression in drought-treated plants.
In the present study, the environmental control of two genes (SRG6, HVA1) whose expression is connected with the development of drought tolerance in barley was studied. SRG6 (stress-responsive gene 6) encodes a putative hydrophobic regulatory protein with a structure typical for the DNA-binding regions of the Helix–loop–Helix (HLH) transcription factors family. Expression of barley SRG6 gene can be induced by ABA and various abiotic stresses like osmotic stress, low temperature and drought, although its role in drought tolerance has not been characterized yet (Malatrasi et al. 2002). A successful transformation of Arabidopsis thaliana using wheat homolog (TaSRG6) was reported, which caused a considerable increase in drought tolerance, resulting in lower relative water loss and higher membrane stability as well as survival rate (Tong et al. 2007). HVA1 encodes a late embryogenesis abundant (LEA) protein, which is synthesized both during cell dehydration caused by water deficit, salt stress, low temperature or under the influence of ABA (Battaglia et al. 2008). The HVA1 gene from barley was used to obtain transgenic plants of rice (Oryza sativa) (Xiao et al. 2007), wheat (Triticum aestivum) (Sivamani et al. 2000) and oat (Avena sativa) (Maqbool et al. 2002), also confirming their increased drought tolerance under field conditions. Both the SRG6 and HVA1 genes were reported as having a higher expression level in barley genotypes more tolerant to drought (Rapacz et al. 2010).
The aim of the present paper was to recognize environmental control of HVA1 and SRG6 transcript accumulation in barley. The effects of soil water deficit, including root to shoot chemical signals, leaf water deficit and light, were studied to verify the hypothesis that different genes important for the drought response may be regulated by different environmental and endogenous signals.
Materials and methods
Plant material
The experiments were performed on four breeding strains of spring two-row barley (H. distichon) selected for different drought tolerance in the previous study (Rapacz et al. 2010). The seeds were obtained from two Polish breeding companies: Danko Plant Breeding, Modzurow branch (MOB12055—susceptible, feed type, Experiment 1 only) and Strzelce Plant Breeding: STH369 (tolerant, feed type, Experiment 1 only), STH754 (tolerant, malt type) and STH836 (susceptible, malt type). MOB12055 and STH754 were reported as having very low and very high yielding potential, respectively, in the dry year 2009, whereas STH369 and STH836 yielded an average level (data from preliminary experiments of breeding companies not published).
Plant growth
The studies were performed in three separate experiments, which were repeated twice. Initial conditions of plant growth were always the same. Plants were grown in a growth room in 6,700 cm3 (37 cm long, 14 cm wide and 13 cm high) pots (one pot per genotype with nine plants each) filled with a mixture of clay, peat and sand (3:2:1, v/v/v) in 50 % air humidity for a 16-h photoperiod and irradiance of 450 μmol m−2 s−1 (provided by high pressure sodium lamps, 400 W; Philips SON-T AGRO, Brussels, Belgium).
The temperature varied according to the developmental stage; namely: during germination (4 days), a constant temperature of 25 °C was maintained, whereas after emergence (also during the drought treatment), the temperature was 25/17 °C (day/night). Plants were watered and fertilized with Florovit multipurpose liquid fertilizer (Inco-Veritas, Gora Kalwaria, Poland). The soil water content was kept at 70 % maximum water capacity (MWC) by adding an appropriate amount of water every day.
Drought treatments
Experiment 1
After reaching the four-leaf stage (18 days from emerging), the watering process was stopped. Air humidity in the chamber was increased from about 50 to 80 % to avoid atmospheric drought. As a result, the water content in the youngest (third) leaves did not change much throughout the entire experiment, whereas the water content in the soil decreased gradually, reaching 60 % of the maximum water capacity (MWC) in the third, 49 % in the fourth, 39 % in the fifth, 35 % in the sixth and finally 31 % (soil water potential −1.99 MPa) on the seventh day of drought treatment. During this period, soil water content was also measured using the HydroSense Soil Water Content Measurement System (Campbell Scientific, Inc., Australia Pty. Ltd.) in different points of the pot in order to check the homogeneity of soil water content. Soil water potential was measured by means of the dew point method using a C-52 thermocouple psychrometer chamber and a HR-33T dew point microvoltmeter (Wescor Inc., Logan, UT, USA). Samples were taken from three places in each pot at a soil layer depth of two-thirds.
Experiment 2
After reaching the same developmental stage as in the first experiment, the youngest but fully developed leaves were detached from the plants and dried at 30 °C in an air drying chamber (Lumel, Zielona Góra, Poland) in the light (400 μmol m−2 s−1, halogen lamp, Osram, Germany) and in the dark.
Experiment 3
The drought treatment approach was the same as in the first experiment with the exception of air humidity in the growth chamber, which was decreased to 50 % to avoid the lack of HVA1 expression in the third leaf, which was observed in Experiment 1 (see results section). In the last day of drought treatment, half the plants were additionally ventilated using a fan in order to increase the transpiration rate, which changed the rate of the decrease in weight of the pots with plants from about 6–10 g/h.
Real-time PCR analysis of SRG6 and HVA1 transcripts accumulation
Samples (0.03–0.05 g from the middle part of the youngest but fully developed leaf) were collected every day of the drought treatment, in the fourth hour of the light period (first experiment) at 0, 55, 110, 165, 220 and 275 min of drying (second experiment). In the third experiment, the samples were collected from the middle part of the first and third leaves on the last day of drought treatment (30 % MWC, −1.99 MPa). There were three biological samples (leaves from two different plants) collected from each genotype studied in each of the two experimental series. Samples were frozen in liquid nitrogen immediately after collection and stored at −80 °C until use. Real-time RT-PCR was used to analyze SRG6 and HVA1 transcript accumulation, as described elsewhere (Rapacz et al. 2010).
Relative quantification of HVA1 and SRG6 transcript copy numbers was performed with the Pfaffl (2001) method using Actin as reference gene. The stability of Actin expression during the experiments was confirmed by means of the variance analysis of its transcript abundance triggered by experimental factors with total cDNA amount in a sample (measured spectrophotometrically, Nanodrop 2000c, ThermoScientific, Wilmington, DE, USA) used for normalization.
Measurements of physiological parameters
All physiological characteristics, except for leaf water potential, were measured during the first experiment every day at the same time (4th–6th hour of the light period), starting from the first day of drought treatment when the MWC was still 70 % (control). Leaf water potential was determined during the second experiment at 55, 110, 165, 220 and 275 min of drying.
Water relations
Relative water content (RWC), water content (WC) and leaf water potential was used to determine the water relations in leaves. The RWC measurements were performed on the first leaves detached from plants in six replications per genotype per day. RWC was calculated as presented by Rapacz et al. (2010). Leaf water potential was measured on the third leaves detached from plants in three replications per genotype at 55, 110, 165, 220 and 275 min of drying (second experiment), according to the dew point method using a C-52 thermocouple psychrometer chamber and a HR-33T dew point microvoltmeter (Wescor Inc., Logan, UT, USA). Water content was measured on the first and third leaves and on stems detached from plants in three replications per genotype (third experiment). WC was calculated according to the equation: WC = 1 − (DW/FW) × 100 %, where FW stands for fresh weight, DW stands for dry weight (determined after 4 days of drying at 30 °C in an air drying chamber—Lumel, Zielona Góra, Poland). Measurements were taken in ten replicates.
Gas exchange
Net photosynthesis (A) and transpiration (E) rates, CO2 concentration inside the leaf (C i) and stomatal conductance (G s) were measured using an infrared gas analyzer (Ciras-1, PP Systems, Hitchin, UK) with a Parkinson leaf chamber (PLC6; PP Systems, Hitchin, UK).
The conditions controlled on the leaf surface during measurements were as follows—temperature: 25 °C; CO2 concentration: 400 μmol(CO2) mol−1(air); irradiance: 500 μmol(quanta) m−2 s−1; relative air humidity: 30 %; air flow rate: 350–400 cm3 min−1. The measurements were repeated in ten individuals.
Photochemical activity of PSII
Chlorophyll a (Chl) fluorescence measurements were used to estimate the photochemical efficiency of PSII. Measurements of Chl fluorescence were performed using the same methods as shown by Rapacz et al. (2010). Photochemical quenching (q p) and the efficiency of excitation energy capture by open PSII reaction centers (F v′/F m′, where F v′ = F 0′ − F m′), as well as the quantum yield of electron transport at PSII (ΦPSII), were calculated according to Genty et al. (1989). The measurements were repeated in ten individuals.
Measurements of biochemical compounds
Abscisic acid
ABA level was evaluated in leaves and stems (between ground level and the first leaf) detached from plants of normal and elevated transpiration. Samples (approx. 0.5 g f.w.) were freeze-dried and ground with a ball mill (Retsch, Kroll, Germany). ABA was extracted with distilled water at 95 °C (1 min) and next at 4 °C (overnight). Extracts were centrifuged for 20 min at 18,000×g, 4 °C (MPW-350R, Poland). ABA was measured in the supernatants using an indirect enzyme-linked immunosorbent assay (ELISA) according to Walker-Simmons and Abrams (1991) with MAC 252 specific antibody (Babraham Technix, Cambridge, UK) and microplate reader Model 680 (Bio-Rad Laboratories, Inc., Munich, Germany). Three independent ELISA measurements were made for each of five biological replications (individuals) of every treatment.
Hydrogen peroxide content
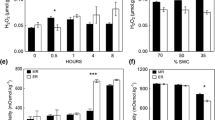
H2O2 was determined as a putative signal molecule in stem segments (between ground level and the first leaf) of the plants with a PeroxiDetect KIT (PD-1), according to the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO, USA). Stem samples (five from each genotype and treatment) were weighed, frozen in liquid nitrogen, homogenized in a TissueLyser2 bead mill (Qiagen, Hilden, Germany) and suspended in 100 μL of cold deionized water and centrifuged at 4 °C at 15,000×g (Centrifuge 5415, Eppendorf, Hamburg, Germany). The final accumulation of Fe3+–xylenol orange complex was measured spectrophotometrically at 560 nm using an Ultrospec 2100 Pro (Amersham Biosciences, Buckinghamshire, UK).
Statistical treatment
Data from all the experiments were analyzed by means of STATISTICA 10.0 software (Statsoft, Tulsa, OK, USA) using GLM model for general effects and Duncan’s test for testing the differences between means at P = 0.05. Genotype and soil water potential were factors in the Experiment 1; genotype, light and leaf water potential in the Experiment 2 and genotype, leaf number and transpiration level were factors in the Experiment 3.
Results
During the first experiment, where the effect of soil water deficit was studied, accumulation of the HVA1 transcript was not observed (data not shown). On the other hand, expression of the SRG6 gene was observed, even under control conditions (soil water potential −0.17 MPa). In all the genotypes studied here SRG6 transcript accumulation was the highest on the sixth day of drought (−1.76 MPa) and a slight further decrease was observed. On the sixth and the seventh day of drought treatment the transcript abundance of SRG6 was much higher in drought tolerant genotypes. In the case of the most drought tolerant (Table 1) genotype (STH754) SRG6 was highly expressed during the whole experiment (even in the control samples).
As shown in Table 1, soil water deficit, together with high air humidity, caused considerable changes in the photochemical activity of PSII (ΦPSII, F v′/F m′, q p). A slight decrease in RWC was visible in two of the lowest soil water potentials, especially in the malt genotypes (STH836, STH754). A clear decrease in stomatal conductance (Gs) was observed when soil water potential decreased to −1.76 and −1.99 MPa, and, at the same time when an increase in SRG6 transcript accumulation was observed (Table 1; Fig. 1). The observed decrease in Gs was the lowest in STH754. Together with closing stomata, a significant decrease in net assimilation (P n) and transpiration (E) rates was observed in all the genotypes with the exception of the most drought tolerant STH754 (Table 1). As a result, at the lowest soil water potential, the highest G s, P n and E values were observed in this genotype. Also, in feed type genotypes, the more tolerant STH369 showed a lower decrease in the assimilation rate than the susceptible MOB12055.
The effect of decreasing soil water potential on changes in SRG6 mRNA copy number in leaves of four barley genotypes relative to MOB12055 at the control conditions—soil water potential −0.17 MPa, using Actin as a reference gene (Experiment 1). Plants were drought treated in the growth chamber at 25/17 °C (day/night), 16-h photoperiod, PAR of 450 μmol m−2 s−1 and 80 % relative air humidity (Experiment 1). Means ± CI for P = 0.05
Two genotypes showing a higher difference in SRG6 transcript accumulation, as well as in physiological reaction to soil water deficit (the malt type: STH754 and STH836), were chosen for further experiments. In the second experiment, detached leaves were dried under controlled conditions. During this experiment, leaf water potential decreased with the drying time, irrespective of light conditions or genotype (Fig. 2). Before the experiment HVA1 transcript accumulation was not detectable in any samples. The drought treatment revealed different expression profiles for the HVA1 gene for drought tolerant (STH754) and susceptible (STH836) genotype. A higher HVA1 transcript accumulation was observed in drought tolerant STH754 than in STH836 (in darkness only) after just 55 min of the treatment, when the water potential of the leaf was decreased for about 0.18 MPa and was stable until the end of the treatment, whereas in the case of the drought susceptible genotype STH836, the accumulation was induced exclusively with the decrease in water potential of about 0.6 MPa and started to decline with further drying (Fig. 2). Light treatment appeared to be a limiting factor for the expression of the HVA1 gene in both of the studied genotypes. In the tolerant genotype, light inhibits the increase in the transcript accumulation in the beginning of the drought treatment only, whereas in the susceptible genotype, it was visible throughout the entire experiment (Fig. 2). The expression of the SRG6 gene increased together with a declining leaf water potential in the drought tolerant genotype (STH754) only, and the highest increase was observed at the end of the experiment (Fig. 3). On the other hand with the exception of the longer drying time in darkness, the expression of SRG6 was even downregulated by the treatment in drought susceptible STH836. Light strongly suppressed the accumulation of the SRG6 transcript in STH754 and this effect was also visible in the end of the experiment for drought susceptible genotype STH836.
The effects of decreasing leaf water potential and light (400 μmol m−2 s−1) during drying the detached leaves of two barley genotypes in an air drying chamber at 30 °C on HVA1 mRNA copy number relative to the copy number observed in STH836 after 55 min of drying in the light using Actin as the reference gene (Experiment 2). Means ± CI for P = 0.05. At time 0 no expression was observed in both genotypes
The effects of decreasing leaf water potential and light (400 μmol m−2 s−1) during drying the detached leaves of two barley genotypes in an air drying chamber at 30 °C on SRG6 mRNA copy number relative to the copy number observed in STH836 in the control (time 0) using Actin as the reference gene (Experiment 2). Means ± CI for P = 0.05
During the third experiment (soil water potential −1.99 MPa, 50 % air humidity), the effect of transpiration rate on the expression of HVA1 and SRG6 genes was measured in the first and the third leaf of STH754 and STH836. A higher accumulation of both transcripts was observed in the drought tolerant (STH754) genotype, irrespective of the leaf age and the transpiration rate of the plants (Table 2). In the case of elevated transpiration rate, the transcript abundance of HVA1 increased in the third leaf of drought tolerant genotype (STH754) only. An increased SRG6 transcript accumulation at elevated transpiration rate was always visible and statistically significant in general, but in the case of drought susceptible STH836 this effect was not statistically significant due to the low level of the expression observed there. In the same experiment water content and ABA accumulation was also measured. There were no differences between genotypes observed in water content (Table 2). The increased transpiration rate did not affect the water content in the leaves, which confirms that the water loss in the leaves of plants with higher transpiration rate was sufficiently supplemented by its transport from the roots. The lower water content was observed in oldest leaves (the first). It appears to be interesting that ABA accumulation was not in accordance with water content in the leaves. Significant differences in ABA accumulation between both genotypes (it was higher in drought tolerant genotype) and transpiration levels was observed only in younger leaves (the third) containing more water and ABA. At elevated transpiration rates a higher ABA accumulation was also observed in stems (Table 3). Thus, it can be supposed that changes in ABA content observed in this experiment can be an element of root to shoot communication. On the other hand, the second potential root to shoot communication particle studied here (H2O2) was only detected in the stem segments of STH836 plants with a normal transpiration rate (Table 3).
Discussion
The aim of this study was to compare the environmental factors and long distance internal signals controlling HVA1 and SRG6 expression in barley leaves during drought treatment. We assumed that because the position of these genes in the chain of reactions of the drought response is quite different, SRG6 being probably a transcription factor and, therefore, taking part in the primary response and HVA1 encoding a protective protein and as such situated in the end of this chain, the factors triggering their expression would not be the same.
Results of the study showed that SRG6 is expressed even in the control samples and that the decrease of water potential in leaves is not required for the further stimulation of SRG6 transcript accumulation. In the Experiment 1, the stimulation of SRG6 transcript accumulation with a steady decrease in soil water potential was observed together with stomata closure without change in RWC of the leaves observed there. In plants growing under conditions of elevated transpiration rate, which contain more ABA but the same amount of water in comparison to the leaves of the same age in plants growing at normal transpiration level, the higher accumulation of SRG6 transcript was observed. This suggested that the expression of SRG6 in drought is caused by a factor regulating stomatal movements that is different from leaf water potential. ABA may be a candidate as it may trigger both stomata closure (Rock 2000) and SRG6 expression as that Tong et al. (2007) proved that the wheat homolog of the SRG6, TaSRG6, is induced in response to exogenous ABA treatment. The measurements of ABA performed in the third experiment and the correlation between SRG6 gene expression and increased ABA level, but not decreased water contents in plants with elevated transpiration rates confirmed that the differences in ABA amounts transported from roots may be indeed responsible for the observed variation in SRG6 expression. Although the further steps in SRG6 expression mediated regulation of plant response to drought remains uncovered our results indicated its role in ABA-dependent response (Rock 2000). In the Experiment 2, the stimulation of SRG6 transcript accumulation was observed only in drought tolerant genotype. In the same experiment no significant changes in ABA contents were observed between either genotypes and time of drying (data not shown), which may suggest that in drought tolerant genotype the expression of this gene can be induced either by the large decrease of leaf water potential or another factor.
The results of our study emphasized that the decrease in intact water potential is more important for triggering high level of HVA1 transcript accumulation than ABA. HVA1 expression was not observed in the Experiment 1, in which no decrease in RWC was observed. The highest rate of the induction of HVA1 expression was observed in Experiment 2, where water deficit was induced in leaves. In the Experiment 3 the level of HVA1 transcript accumulation was much higher in leaves with lower water (but higher ABA) contents. The inductive effect of ABA content on the HVA1 expression observed in the same experiment in the case of high water containing leaves of tolerant genotype only was fourteen times lower than the effect of water deficit observed in the same plants.
It was reported that ABA induced HVA1 expression level in the barley aleurone layer (Hong et al. 1988). Shen et al. (1996) found the abscisic acid response complex (ABRC) in the structure of the HVA1 promoter which is necessary and sufficient for ABA-dependent induction of gene expression. Casaretto and Ho (2003) discovered the appropriate transcription factors required for ABA induction of HVA1 expression in aleurone cells. All these papers showed that the HVA1 gene expression in aleurone cells is strongly induced by ABA in vitro, and it is also assumed that this happens in vivo in the rest of the plant exposed to drought. Also in our study ABA may affect the level of HVA1 transcript accumulation in leaves in which water potential was not affected by drought, but this effect was much lower than the effect of the decrease in leaf water potential per se. Hong et al. (1992) suggested that the expression of HVA1 is under developmental regulation, based on experiments with ABA induction of its expression in the vegetative tissues of plants of different ages. Maybe the role of ABA as a triggering signal for HVA1 expression decreases with the age of the plant, being replaced by other driving forces.
H2O2 assumed in the present paper as the second drought-induced root to shoot chemical signal was not recognized as a factor responsible for the regulation of both HVA1 and SRG6 gene expression as well as stomatal closure. The accumulation of H2O2 in stems was observed only in the less tolerant genotype under conditions of low transpiration rate, which indicates that the observed accumulation may rather be the effect of oxidative stress taking place during drought. The higher level of H2O2 in leaves was observed in the light (data not shown), where the expression of both genes studied was lower. Thus, the putative role of hydrogen peroxide and/or the increased over-reduction of PSII in the down-regulation of both genes in the light cannot be excluded, as was suggested before on the basis of correlations observed between the chlorophyll fluorescence based characteristics of PSII and the transcript accumulation levels (Rapacz et al. 2010). The negative effect of light on the transcript accumulation of genes studied in the present paper may be connected with the presumed mechanism preventing drought response under conditions of temporary and harmless water deficits occurring at midday as a result of the temporary high water deficit in the air. However, an unambiguous explanation of the molecular mechanism of this regulation is impossible at this stage of the research.
The results of our study indicate that the expression of both SRG6 and HVA1 was higher in genotypes in which many of the physiological characteristics (water status, gas exchange, photochemical performance of PSII, cellular membranes stability) were less sensitive to drought (Rapacz et al. 2010), thereby confirming the role of these genes in drought response as important elements of this complex mechanism (Xu et al. 2010; Sivamani et al. 2000; Tong et al. 2007). In the study of Rapacz et al. (2010), two groups of barley genotypes (feed and malt types) were analyzed separately for various physiological and molecular characteristics connected with drought tolerance, including SRG6 and HVA1 transcript accumulation. Although the highest level of accumulation of both transcripts was observed in most tolerant genotypes from both groups, only the HVA1 expression level in more tolerant malting barleys was clearly correlated with higher photosynthetic activity in drought, whereas in the less tolerant fodder group, other traits were responsible for differences in the drought tolerance observed between genotypes. This result, together with the results of the present paper, may suggest that the accumulation of HVA1 is the last defense against drought, which can ensure relative high photosynthetic productivity when leaf water potential decreases. On the other hand, this mechanism seems to be insufficient for the development of drought tolerance in genotypes with other mechanisms not working properly, which was, in general, observed in the group of feed barleys studied previously by Rapacz et al. (2010).
Tong et al. (2007) showed that the expression of SRG6 homolog, TaSRG6, is induced early after the stress treatment, which led him to conclusion that it may be a transcription factor triggered by abiotic stresses. The present study revealed that in contrast to HVA1, SRG6 is expressed constitutively in plant leaves, and its expression is increased in drought. It may indicate that SRG6, apart from its role in the drought tolerance mechanism, may have another, as yet undefined function.
On the basis of the present results, as well as of the previous study by Rapacz et al. (2010), a simplified model of the drought response may be suggested for the tolerant STH754 barley genotype. This was reported as the most drought tolerant in the previous study and had the best yield under drought conditions in multisite preliminary experiment located in Poland (data not shown). STH754 responds to soil drought with a high level of ABA signal coming from the roots to the leaves. This signal triggers the expression of the regulatory SRG6 gene, and the high level of this expression is also unique to this genotype; however, the exact function of the SRG6 protein is unknown. On the other hand, stomata of this genotype are not sensitive to the ABA signal coming from roots, which ensures a high level of net photosynthesis under moderate drought. And finally, when leaf water deficit increases, the expression of the HVA1 gene, which encodes a dehydration protective protein, is induced here to a level much higher than in other genotypes.
It is interesting that although in the case of both genes studied here different internal factors are predominant for triggering gene expression (water deficit for HVA1 and ABA for SRG6); some effect of the second of them was also visible but only in the case of drought tolerant genotype. Thus, it seems to be possible that in drought tolerant genotypes HVA1 and SRG6 are not only more expressed during drought but tolerant genotypes are more sensitive to various internal signals confirming environmental water deficit.
In conclusion, although both SRG6 and HVA1 are involved in the drought response of barley, their expression seems to be controlled by partially different signals. Additionally, the level of both transcript accumulation is additionally reduced in the light. Because of this powerful complexity, a true understanding of plant reaction to drought requires further studies integrating gene expression and cell signaling analysis in single organs or tissues with whole plant physiology and long distance signaling. Such studies would be most valuable when performed under field conditions, where local soil and atmospheric conditions may modulate drought response.
Author contribution
M. Wójcik-Jagła contributed to all the experimental process, data analysis and results interpretation as well as paper preparing. W. Barcik was involved in qRT-PCR, ABA and physiological analysis. F. Janowiak was responsible for experimental design for ABA root to shoot communication study as well as for ABA immunoassay. M. Rapacz coordinated experimental process, was responsible for statistical treatment, figure drawing and contributed to the preparing of final manuscript version.
References
Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148:6–24
Bian S, Jiang Y (2009) Reactive oxygen species, antioxidant enzyme activities and gene expression patterns and recovery. Scientia Horticulare 120:264–270
Boudsocq M, Laurière C (2005) Osmotic signaling in plants. Multiple pathways mediated by emerging kinase families. Plant Physiol 138:1185–1194
Casaretto J, Ho T-HD (2003) The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell 15:271–284
Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Marè C, Tondelli A, Stanca AM (2008) Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crop Res 105:1–14
Foyer CH, Noctor G (2005) Oxidant and antioxidant signaling in plants: a revaluation the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Hong B, Uknes JS, Ho T-HD (1988) Cloning and characterization of a cDNA encoding a mRNA rapidly-induced by ABA in barley aleurone layers. Plant Mol Biol 11:495–506
Hong B, Barg R, Ho T-HD (1992) Developmental and organ-specific expression of an ABA- and stress-induced protein in barley. Plant Mol Biol 18:663–674
Jana S, Wilen RW (2005) Breeding for abiotic stress tolerance in barley. In: Ashraf M, Harris PJC (eds) Abiotic stresses. Plant resistance through breeding and molecular approaches. Haworth Press, New York, pp 491–511
Malatrasi M, Close TJ, Marmirolli N (2002) Identification and mapping of putative stress response regulator gene in barley. Plant Mol Biol 50:143–152
Maqbool SB, Zhong H, El-Maghraby Y, Ahmad A, Chai B, Wang W, Sabzikar R, Sticklen M (2002) Competence of oat (Avena sativa L.) shoot apical meristems for integrative transformation, inherited expression, and osmotic tolerance of transgenic lines containing hva1. Theor Appl Genet 105:201–208
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pfannschmidt T, Bräutigam K, Wagner R, Dietzel L, Schröter Y, Steiner S, Nykytenko A (2009) Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Ann Bot 103:599–607
Rapacz M, Kościelniak J, Jurczyk B, Adamska A, Wójcik M (2010) Different patterns of physiological and molecular response to drought in seedlings of malt- and feedtype barleys (Hordeum vulgare). J Agron Crop Sci 196:9–19
Rock CD (2000) Pathways to abscisic acid-regulated gene expression. New Phytol 148:357–396
Schachtman DP, Goodger JQD (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287
Shen Q, Zhang P, Ho T-HD (1996) Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell 8:1107–1119
Sivamani E, Bahieldin A, Wraith JM et al (2000) Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci 155:1–9
Tong S, Ni Z, Peng H, Dong G, Sun Q (2007) Ectopic overexpression of wheat TaSRG6 gene confers water stress tolerance in Arabidopsis. Plant Sci 172:1079–1086
Tuberosa R, Salvi S (2006) Genomics-based approaches to improve drought tolerance of crops. Trends Plant Sci 11:405–412
Vandenbroucke K, Robbens K, Vandepoele K, Inzé D, Van de Peer Y, Van Breusegem F (2008) Hydrogen peroxide–induced gene expression across kingdoms: A comparative analysis. Mol Biol Evol 25:507–516
Walker-Simmons MK, Abrams SR (1991) Use of ABA immunoassays. In: Davies WJ, Jones HG (eds) Abscisic acid, physiology and biochemistry. Bios Scientific Publishers, Oxford, pp 53–63
Xiao B, Huang Y, Tang N, Xiong L (2007) Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor Appl Genet 115:3546
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654
Yang S, Huang C, Wu Z (2006) Stomatal movement in response to long distance-communicated signals initiated by heat shock in partial roots of Commelina communis L. Sci China Ser C Life Sci 49:18–25
Acknowledgments
This work was supported by Polish National Research and Development Center [PBZ-MNiSW-2/3/2006/17].
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Aroca.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wójcik-Jagła, M., Rapacz, M., Barcik, W. et al. Differential regulation of barley (Hordeum distichon) HVA1 and SRG6 transcript accumulation during the induction of soil and leaf water deficit. Acta Physiol Plant 34, 2069–2078 (2012). https://doi.org/10.1007/s11738-012-1004-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1004-0