Abstract
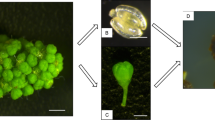
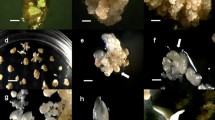
A repeatable in vitro culture method was established and it could induce a series of abnormal embryos by which their scale leaves were substituted by petals. These petal-bearing embryos were derived from long-term root calli of an orchid cultivar, Oncidium ‘Gower Ramsey’. The calli were induced and subcultured on a modified 1/2MS medium supplemented with five combinations of TDZ and dicamba. When 1-year-old callus, which induced and subcultured at 3 mg/l TDZ and 5 mg/l dicamba (line 13 callus), was transferred onto 1/2MS medium supplemented with 0.1 ml/l NAA and 3 mg/l TDZ, it gave the highest number of petal-bearing embryos. However, line 13 root explants gave one of the lowest percentage of callus formation (12.5%) and the number of somatic embryos per callus was also one of the lowest (along with lines 10, 11 and 12). The efficiency of embryogenesis decreased with age of callus and the significant decrease was started from the third year of culturing. Flowering characteristics of plantlets from normal embryos and petal-bearing embryos were both evaluated after 3 years of culture in the greenhouse. Parameters including length of the longest inflorescence, numbers of flowers per plant, length of flowers and width of flowers were all not significantly different between abnormal embryo-derived plants and normal embryo-derived plants.





Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- 2iP:
-
N 6-2-Isopentenyl adenine
- BA:
-
N 6-Benzyladenine
- GA3 :
-
Gibberellin A3
- IBA:
-
Indole-3-butyric acid
- Kinetin:
-
N 6-Furfuryladenine
- Dicamba:
-
3,6-Dichloro-2-methoxybenzoic acid
- MS:
-
Murashige and Skoog (1962) medium
- NAA:
-
1-Naphthaleneacetic acid
- PGR:
-
Plant growth regulator
- TDZ:
-
1-Phenyl-3-(1,2,3-thiadiazol-5-yl)-urea
References
Arditti J, Ernst R (1993) Micropropagation of orchids. John Wiley & Sons, Inc., New York
Chang C, Chang WC (2003) Cytokinins promotion of flowering in Cymbidium ensifolium var. misericors in vitro. Plant Growth Regul 39:217–221
Chang WC, Hsing YI (1980) In vitro flowering of embryoids derived from mature root callus of ginseng (Panax ginseng). Nature 284:341–342
Chen JT, Chang WC (2000a) Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (Orchidaceae). Plant Sci 160:87–93
Chen JT, Chang WC (2000b) Plant regeneration via embryo and shoot bud formation from flower-stalk explants of Oncidium Sweet Sugar. Plant Cell Tissue Organ Cult 62:95–100
Chen JT, Chang C, Chang WC (1999) Direct somatic embryogenesis and subsequent plant regeneration from leaf explants of Oncidium ‘Gower Ramsey’. Plant Cell Rep 19:143–149
Chia TF, Arditti J, Segeren M, Hew CS (1999) Review: in vitro flowering of orchids. Lindleyana 14:60–76
Compton ME (1994) Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tissue Organ Cult 37:217–242
Dawns CJ (1971) Biological techniques in electron microscopy. Barnes and Noble, New York
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Goldberg RB, de Paiva G, Yadegari R (1994) Plant embryogenesis: zygote to seed. Science 266:605–614
Guo B, Abbasi BH, Zeb A, Xu LL, Wei YH (2011) Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotech 10:8984–9000
Hee KH, Loh CS, Yeoh HH (2007) In vitro flowering and rapid in vitro embryo production in Dendrobium Chao Praya Smile (Orchidaceae). Plant Cell Rep 26:2055–2062
Immonen AST (1996) Influence of media and growth regulators on somatic embryogenesis and plant regeneration for production of primary triticales. Plant Cell Tissue Organ Cult 44:45–52
Jung C, Müller AE (2009) Flowering time control and applications in plant breeding. Trends Plant Sci 14:563–597
LeRoux G, Barabé D, Vieth J (1997) Morphogenesis of the protocorm of Cypripedium acaule (Orchidaceae). Plant Syst Evol 205:53–72
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:495–497
Nishimura G (1981) Comparative morphology of Cattleya and Phalaenopsis (Orchidaceae) seedings. Bot Gaz 142:360–365
Osuna P, Barrow JR (2004) Regeneration of black grama (Bouteloua eriopoda Torr. Torr) plants via somatic embryogenesis. In Vitro Plant 40:299–302
Płażek A, Filek M, Wędzony M (1999) Improvement of regeneration ability in Phleum pratense L. in vitro culture by dicamba. Acta Physiol Plant 21:397–403
Sim GE, Loh CS, Goh CJ (2007) High frequency early in vitro flowering of Dendrobium Madame Thong-In (Orchidaceae). Plant Cell Rep 26:383–393
Smulders MJM, de Klerk GJ (2011) Epigenetics in plant tissue culture. Plant Growth Regul 63:137–146
Soltis DE, Soltis PS, Albert VA, Oppenheimer DG, dePamphilis CW, Ma H, Frohlich MW, Theissen G (2002) Missing links: the genetic architecture of flower and floral diversification. Trends Plant Sci 7:22–31
Steinmacher DA, Clement CR, Guerra MP (2007) Somatic embryogenesis from immature peach palm inflorescence explants: towards development of an efficient protocol. Plant Cell Tissue Organ Cult 43:124–132
Von Arnold S, Sabala I, Bozhkov I, Dyachok J, Filonoval L (2005) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Wang HC, Chen JT, Chang WC (2010) Morphogenetic routes of long-term embryogenic callus culture of Areca catechu. Biol Plant 54:1–5
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Borkowska.
Rights and permissions
About this article
Cite this article
Chen, JT. Induction of petal-bearing embryos from root-derived callus of Oncidium ‘Gower Ramsey’. Acta Physiol Plant 34, 1337–1343 (2012). https://doi.org/10.1007/s11738-012-0930-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-0930-1