Abstract
High productive suspension cultures of Genista tinctoria were elicited (methyl jasmonate or chitosan) and permeabilised (dimethyl sulfoxide) in order to achieve a plant in vitro system rich in isoflavones, with extracellular storage profile. The maximum concentration of isoflavone aglycones (4–6 times higher than in the controlled biomass) was obtained in the suspension elicited with chitosan. All isoflavonoids were stored inside cells. In case of methyl jasmonate supplementation the total concentration of isoflavone aglycones achieved was the result of the de novo biosynthesis as well as a hydrolysis of the storage ester forms. The presence of chitosan in the medium was only associated with the production of aglycones de novo. The elicitors had no effect on the accumulation and metabolism of the basic glycoside isoflavones in the suspension. The change in the way the isoflavones were stored was achieved after supplementing the growth media with dimethyl sulfoxide. Maximum concentration of isoflavones in the medium was observed in the 7th hour of the experiment. Eventually, a plant growth system was developed, producing over 11% of isoflavones, ejected as a result of chemical permeabilisation, into the growth medium (approx. 80% of total amount of these compounds in the biomass).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the variety of retrobiosynthetic strategies used for in vitro cultures of higher plants oriented on the secondary metabolite biosynthesis, the ones which need mentioning include elicitation of plant biomasses which can directly or indirectly affect the accumulation and metabolism of secondary metabolites (Bhuiyan and Adachi 2003; Bourgaud et al. 2001; Chang-He and Hui-Bi 2001; Ramachandra Rao and Ravishankar 2002; Staniszewska et al. 2003).
Biotechnological research, previously carried out by the authors of this paper, showed that G. tinctoria suspensions are able to synthesise one of the largest amounts of isoflavonoids of phytoestrogenic activity ever obtained in vitro (Luczkiewicz and Głód 2005). Considering their intensive growth (FWmax 748.6 g l−1) and high isoflavonoid accumulation (ca. 11%, of which 9414.7 mg/100 g DW comprise genistin), they may be seen as a potential in vitro system for industrial scale production of large amounts of medically active phytoestrogens of isoflavone origin (Luczkiewicz and Głód 2005).
Isoflavones due to their affinity to oestrogen receptors β, in addition to treatment of menopause-related symptoms, may prevent breast, colon, prostate and thyroid cancers which are hormone related (Brandi 1997; Dixon and Ferreira 2002; Radzikowski et al. 2004).
Most compounds identified in the 14-component isoflavonoid fraction in G. tinctoria suspension are phytoalexins or phytoanticipins (Luczkiewicz and Głód 2005), in the biosynthesis of which a considerable role could be played by stress factors (Barz and Mackenbrock 1994; Edwards et al. 1997; Paiva et al. 1994). Based on a number of literature data it can be stated that both biotic and abiotic elicitors have a particular effect on the accumulation of isoflavones in in vitro cultures of Fabaceae plants (Barz and Mackenbrock 1994; Bhuiyan and Adachi 2003; Bourgaud et al. 2001; Edwards et al. 1997; Gagnon and Ibrahim 1997; Paiva et al. 1994; Ramachandra Rao and Ravishankar 2002).
Considering the above, the direct goal of the paper was to give an answer to the question whether the addition of certain elicitors may evoke higher concentration of various isoflavonoid metabolites in G. tinctoria biomass, compared with the basic suspension, and whether the procedure applied changes the way in which the analysed compounds are stored. In this experiment, the elicitors of choice were methyl jasmonate and chitosan. These compounds may be considered model elicitors as they are frequently used in experiments testing the effect of stress conditions on the metabolism of isoflavones in in vitro cultures of Fabaceae plants (Barz and Mackenbrock 1994; Bednarek et al. 2001; Bhuiyan and Adachi 2003; Edwards et al. 1997; Gagnon and Ibrahim 1997; Paiva et al. 1994; Ramachandra Rao and Ravishankar 2002).
In G. tinctoria suspension cultures all of the synthesised isoflavonoids were stored intracellularly (Luczkiewicz and Głód 2005). To upgrade the G. tinctoria biomass to large-scale production, a decision was made to evoke a release of the investigated compounds from the plant matrix to the growth medium, thus simplifying the subsequent extraction procedures. This would reduce the costs of industrial production of phytoestrogens based on G. tinctoria suspension (Bourgaud et al. 2001; Luczkiewicz and Głód 2005; Ramachandra Rao and Ravishankar 2002). The intention was to stimulate a release of isoflavonoids to the experimental medium by chemical permeabilisation of cellular membranes in G. tinctoria suspension (Bordelius 1988; Bourgaud et al. 2001; Knorr and Berlin 1987; Komolpis et al. 1998; Ramachandra Rao and Ravishankar 2002). The direct permeabilising factor in the described series of experiments was dimethyl sulfoxide (DMSO), which had been successfully used to evoke a release of secondary metabolites from plant matrices grown in vitro (Bordelius 1988; Kanaraiah et al. 2005; Komolpis et al. 1998).
The success of the planned experiments, i.e. the further improvement of productivity of the culture as a direct result of elicitation procedure and causing a release of isoflavones into the growth medium would constitute an essential step towards preparing G. tinctoria suspension for the technological process.
Materials and methods
Plant material
Highly productive suspension cultures of G. tinctoria cultivated in modified, liquid Schenk–Hildebrandt medium (SH) (Schenk and Hildebrandt 1972) containing 3% (w/v) sucrose, 1.0 μmol l−1 TIBA (2,3,5-triiodobenzoic acid) and 23.23 μmol l−1 kinetin (SHz) (Luczkiewicz and Głód 2005) were used for all biotechnological experiments. Plant biomasses were grown in 250 ml Erlenmeyer flasks topped with silicone foam stoppers (Shine-Etsu-Polymer Co., Japan) on an orbital shaker (INNOVA 2300-New Brunswick Scientific 150 rpm) under continuous light (light intensity 88 ± 8 μmol m−2 s−1; fluorescent tubes Philips TLD 35 W ×3), at temp. 25 ± 2°C (Luczkiewicz and Głód 2005).
Elicitor solutions
Chitosan
1.0 g of crab shell chitosan (Sigma Chemical Company, USA) was dissolved in 2.0 ml of glacial acetic acid (J.T. Baker, USA) adding the reagent in drops at 60oC for a period of 15 min. and the final volume was made up to 100 ml. The pH of the solution was adjusted to 5.7 with 1 N NaOH (J.T. Baker, USA). Prior to adding it to the G. tinctoria suspension, the solution of chitosan was autoclaved in standard conditions (Komaraiah et al. 2002).
Methyl jasmonate
The basic solution of the elicitor was prepared by dissolving 100 mg of methyl jasmonate (Sigma Chemical Company, USA) in 100 ml of the following mixture: ethanol (J.T. Baker, USA)—redistilled water (12 : 3, v/v) (Bhuiyan and Adachi 2003). Before being added to the experimental media with inoculated (day 0 of the experiment) or actively growing (day 26 of the experiment) G. tinctoria biomass, the elicitor was filter sterilised (Millex Vent Filter, pore size 0.22 μm, PTFE membrane; Millipore Corporation, Bedford, USA).
Experimental procedures
Elicitation
In each instance, approx. 5 g of basic G. tinctoria suspension was inoculated into a 250 ml Erlenmeyer flask containing 100 ml of modified, liquid SH medium (SHz) topped with silicone foam stoppers. Both elicitors (chitosan 150 mg l−1 and methyl jasmonate 100 μmol l−1) were added to the media on day 0 or 26 of the growth cycle. The elicited suspension cultures of G. tinctoria were cultivated in a 34-day cycle (3 series, 22 flasks each), under standard conditions described above for the basic suspension.
G. tinctoria biomasses were collected at two-day intervals until day 26 of the experiment and then each day until the end of the growth cycle (34 days altogether).
The regular control samples in these experiments were basic G. tinctoria suspensions grown in SHz medium without elicitors. The negative controls with acetic acid and ethanol/redistilled water (12:3 v/v) solutions were accomplished too. Both controls were collected using the same procedure as for the elicited cultures. The results of regular and negative controls in terms of isoflavones production did not differ significantly so only the first ones were presented in the manuscript.
Permeabilisation
The direct permeabilisating factor was DMSO (Sigma Chemical Company, USA) added in the amount of 20 ml l−1 to the actively growing G. tinctoria suspension.
In each instance, approx. 5 g of basic G. tinctoria suspension was inoculated into a 250 ml Erlenmeyer flask containing 100 ml of SHz medium. The biomasses were cultivated in standard conditions for 26 days. In order to evoke a release of isoflavones from the plant matrix to the growth medium, on day 26 of the experiment the media were supplemented with a single dose of DMSO (20 ml l−1) (Bordelius 1988; Kanaraiah et al. 2005). The permeabilised suspensions of G. tinctoria were grown in the conditions described above for the basic suspensions, collecting the biomass at 30 min. intervals during a period of 8 h. (3 series, 17 flasks each).
The control samples in this case were G. tinctoria suspensions grown in SHz medium without DMSO in standard conditions and collected in 30-min. intervals like the permeabilised biomasses.
The plant material obtained in the course of the biotechnological experiments, together with the liquid media (basic, elicited and permeabilised cultures) were freeze-dried for 48 h. (vacuum 1 × 10−3 mbar produced in the lyophyliser—LYOVAC GT2 by FIN-AQUA Santasalo-Sohlberg Co., Finland). The lyophilised G. tinctoria biomasses and growth media were analysed phytochemically for the presence of bioflavonoids. The results (fresh weights—FW, dry weights—DW and the isoflavones content (in the plant material and the media) are arithmetic means from three samples ±SD.
Isoflavonoid extraction from plant materials and media, together with HPLC qualitative and quantitative analyses were performed according to the procedures described before (Luczkiewicz et al. 2004).
Results and discussion
The main goal of the described experiments was to check whether biotechnological strategies related to elicitation and permeabilisation of highly productive G. tinctoria suspension (11,138.5 mg of isoflavones in 100 g DW) (Luczkiewicz and Głód 2005) could affect the accumulation and the storage profile of isoflavones in the investigated biomass.
Based on literature reports, two elicitors were selected for the experiments presented here: methyl jasmonate and chitosan, which yield good results in in vitro cultures of higher plants oriented for the biosynthesis of biologically active compounds (Barz and Mackenbrock 1994; Bednarek et al. 2001; Bourgaud et al. 2001; Ramachandra Rao and Ravishankar 2002).
As the preliminary results of experiments involving permeabilisation of G. tinctoria suspension with physical factors (radical changes of temperature, electrical impulses) yielded all negative results (isoflavonoids not released into the media), it was decided to present only the effect of chemical permeabilisation of G. tinctoria biomass on the generally understood accumulation of isoflavones. Based on literature reports the direct permeabilising factor used was dimethyl sulfoxide (DMSO) at the concentration of 20 ml l−1 (Komolpis et al. 1998).
The effect of a methyl jasmonate supplement on the growth of G. tinctoria suspension and the accumulation of isoflavonoid compounds
Methyl jasmonate was added to G. tinctoria suspension on day 0 or day 26 of the experiment. Thus, it was possible to follow the effect of this elicitor on the growth of G. tinctoria biomass and the kinetics of isoflavone production over the entire 34-day growth cycle (the addition of jasmonate to the inocular suspension) or just until the moment when the suspension reaches a stationary growth phase and accumulates maximum concentrations of isoflavonoids (day 26) (Luczkiewicz and Głód 2005). Thanks to the described procedure of feeding G. tinctoria suspension with methyl jasmonate on day 26, an optimum system of elicitation was developed for the investigated biomass, allowing for the stimulation of the isoflavonoid biosynthesis, while excluding any negative effects of the elicitor on the culture growth (Tables 1, 2, 3; Figs. 1, 2, 3, 4).
The fresh weight (FW) and dry weight (DW) measured on the particular days of the experiment indicate that methyl jasmonate clearly inhibited the growth and vitality of G. tinctoria culture. The described phenomenon is particularly apparent when the elicitor was added to the inocular suspension (Figs. 1 and 2).
Unlike the control biomass, the cultures supplemented with methyl jasmonate practically did not grow until day 16 (FW 49.5 g l−1; Fig. 1) and then gradually died (FW 28.1 g l−1, day 34; Fig. 1). In effect, on day 22 of the experiment, when FW of the basic suspension was at the maximum level (FW 748.6 g l−1), the elicited culture produced only 47.6 g l−1 of the biomass, i.e. almost 16 times less than the control culture (Fig. 1).
The growth inhibition of G. tinctoria suspension, as a direct effect of methyl jasmonate supplementation, is less apparent in the case of biomass supplemented with the elicitor on day 26 of the experiment (Figs. 1 and 2). This could be related to the relatively short period of growing G. tinctoria suspension with the elicitor (9 days) and the fact that in the discussed instance the analysed culture was in the stationary growth phase (Figs. 1 and 2).
As a result, a number of literature reports were confirmed, where the researchers noted a clear reduction of both FW and DW in plant cultures elicited with methyl jasmonate or jasmonic acid (Barz and Mackenbrock 1994; Bhuiyan and Adachi 2003; Komaraiah et al. 2002; Paiva et al. 1994). It is commonly believed that jasmonic acid, often classified as an endogenous growth regulator (Ebdel and Mithöfer 1998; Ozawa et al. 2000; Schenk et al. 2000), inhibits tissue growth and accelerates ageing processes in plant biomasses (Barz and Mackenbrock 1994; Bhuiyan and Adachi 2003; Komaraiah et al. 2002; Paiva et al. 1994).
G. tinctoria suspension elicited with methyl jasmonate synthesised a set of isoflavones, identical with the compounds identified in the control culture (Luczkiewicz and Głód 2003, 2005). The basic components of the isoflavonoid fraction were derivatives of genistein (genistin, genistein 7-O-diglucoside, 2′-hydroxygenistein 7-O-glucoside and genistin malonate) with significantly lower concentration of compounds which belong to the metabolic pathway of daidzein (Tables 1, 2, 3). The supplementation of G. tinctoria suspension with methyl jasmonate did not affect the way the investigated compounds were stored. Similar to the basic culture, all isoflavonoids were stored intracellularly.
At this stage of the experiments it is difficult to explain why the synthesised metabolites are not released from the elicited suspension into the medium, which was often observed in in vitro cultures subjected to stress (Armero et al. 2001; Bourgaud et al. 2001; Pitta-Alvarez et al. 2000).
The supplementation of G. tinctoria suspension with methyl jasmonate on inoculation day did not show any effect of the elicitor on the accumulation of isoflavonoids in the investigated biomass. This is probably directly connected with the fast biomass necrosis observed in the experiment. In effect, the concentrations of all analysed isoflavonoid compounds in G. tinctoria suspension were at the levels identified in the inocular biomass (Table 2).
Elicitation of G. tinctoria cultures on day 26 of the experiment had a selective effect on the accumulation of isoflavonoid aglycones (genistein and daidzein) and genistein malonate (Table 3 and Figs. 3, 4). Beginning with the day when methyl jasmonate was supplemented, the concentrations of both aglycones gradually rose, reaching maximum levels on day 30 (198.3 mg/100 g DW genistein; 15.21 mg/100 g DW daidzein; Table 3). Thus, the maximum concentration of genistein in the elicited culture was three times higher than in the control biomass (Tables 1, 3 and Fig. 4), and in the case of daidzein it exceeded five times the level of the compound identified in the basic suspension (Tables 1, 3 and Fig. 3). It was also found that the concentration of genistin malonate in the culture decreased 30 times in the described time frame (69.4 mg/100 g DW—day 30—control biomass; 2.5 mg/100 g DW—day 30—elicited suspension; Tables 1 and 3). The other isoflavonoids in the elicited suspension remained at the same concentrations as in the control culture (Tables 1 and 3).
The results of these experiments are consistent with other reports which indicate that under stress plants selectively synthesise isoflavonoid aglycones which serve as phytoalexins (Armero et al. 2001; Barz and Mackenbrock 1994; Edwards et al. 1997; Gagnon and Ibrahim 1997; Haberder et al. 1989; Paiva et al. 1994). This process is related to de novo biosynthesis of these compounds, or it is the result of hydrolytic decomposition of esters which play the role of phytoanticipins (Armero et al. 2001; Barz and Mackenbrock 1994; Lozovaya et al. 2004).
High concentrations of genistein as a result of biomass elicitation, in conjunction with clear decomposition of the storage form of phytoanticipin, indicate that the reaction to stress in G. tinctoria cultures may be rather complex (Armero et al. 2001; Lozovaya et al. 2004). It is most probably related not only to the de novo biosynthesis of phytoalexins, but also to the indirect formation of genistein as a result of hydrolytic decomposition of genistein malonate.
The secondary formation of isoflavonoid aglycone (genistein) took place without the participation of glycosides (concentrations of these compounds in the elicited culture were the same as the content of the respective metabolites in the control biomass—Tables 1 and 3).
In the growth process (a 34-day growth cycle), the rapid decrease in the content of genistein and daidzein, paired with secondary growth of the ester concentration (Table 3; Fig. 4), i.e. the phenomenon frequently seen in elicited in vitro cultures of Fabaceae plants, was not observed (Barz and Mackenbrock 1994; Lozovaya et al. 2004). Perhaps in G. tinctoria biomass the defence mechanism, which in this case, as in Pueraria lobata suspension (Park et al. 1995) had the character of a systemic reaction due to the constant contact of individual cells with the elicitor, was not phased out (Ebdel and Mithöfer 1998; Haberder et al. 1989; Park et al. 1995).
In effect, the application of the biotechnological procedure described above, a highly productive suspension of G. tinctoria was obtained, which synthesised isoflavonoid glycosides at the level of the control culture and, at the same time, accumulated several times higher concentrations of aglycones (Tables 1 and 3).
The effect of chitosan on the growth of G. tinctoria suspension and the accumulation of isoflavonoids
Isoflavones synthesised in suspension cultures of G. tinctoria were stored inside the cells (Luczkiewicz and Głód 2005). A leak of these compounds into the growth medium would significantly facilitate the procedure of extracting isoflavones from the plant matrix. Based on literature reports (Bhuiyan and Adachi 2003; Komolpis et al. 1998; Ozawa et al. 2000), it was expected that chitosan added to G. tinctoria suspension would play a double role, both as elicitor and a factor permeabilising the investigated cells.
The experiment confirmed that chitosan had a significant impact on both the growth of the investigated suspension (Figs. 1 and 2) and the accumulation of isoflavonoids (Tables 2, 3 and Figs. 3, 4). Like methyl jasmonate, this elicitor inhibited the growth and vitality of the propagated biomass when added to the media on the inoculation day (Figs. 1 and 2). Beginning with the first day of the experiment, over the entire 34-day growth cycle, the culture grown in the presence of chitosan demonstrated a gradual decrease of both DW and FW (Figs. 1 and 2). In effect, on the last day of the experiment 30.4 g l−1 FW of G. tinctoria suspension was obtained, which was only ca. 60% of the starting value (50.3 g l−1 FW, Fig. 1).
The growth profile of G. tinctoria suspension, elicited with chitosan, in the stationary growth phase (day 26) was almost completely identical with the growth curve established for the control culture (Figs. 1 and 2). These results were similar to the effects achieved when the culture was supplemented with methyl jasmonate and indicate that irrespective of the stress-inducing factor, it is essential to supplement the elicitor at a specific stage of the in vitro experiment, i.e. in this case in the stationary phase. It seems that the use of the above experimental procedure allows to avoid the negative impact of the elicitor on primary tissue metabolism (Chang-He and Hui-Bi 2001; Ebdel and Mithöfer 1998; Komaraiah et al. 2002) and to obtain only positive effects of elicitation, related to the selective influence of the stress factor on secondary metabolism (Figs. 3 and 4).
Supplementing G. tinctoria suspension with chitosan did not cause a change in the qualitative composition of the isoflavonoid fraction synthesised by the investigated culture. However, the time of elicitation proved important, as it directly affected the amount of accumulated compounds. Like with methyl jasmonate, chitosan added to the media on the day of inoculation generally inhibited the production of isoflavonoids in the biomass (Tables 1, 2, 3). In both cases the effect was correlated with the necrotic tendencies of the suspension (Figs. 1 and 2).
The elicitation of G. tinctoria biomass with chitosan on day 26 selectively increased the concentration of isoflavonoid aglycones (daidzein and genistein) in the investigated suspension (Table 3 and Figs. 3, 4). As in the cultures supplemented with methyl jasmonate, after the elicitor was added, the concentrations of both compounds gradually rose, reaching maximum levels on day 30 (258.4 mg/100 g DW genistein; 17.51 mg/100 g DW daidzein) and then stayed at a relatively steady level (Table 3).
The high concentration of isoflavonoid phytoalexins accumulated in the suspension was probably fully related to de novo biosynthesis of these compounds. One indication to this effect is that the concentration of glycoside derivatives of genistein and daidzein in the biomass remained steady in relation to the control biomass (Tables 1 and 3). Moreover, in contrast to the experiment with methyl jasmonate, the level of the phytoalexin storage form (genistin malonate) was gradually growing over the entire growth cycle, reminding the production of this compound in the basic culture (Tables 1, 3 and Fig. 4). The phenomenon described here seems to rule out the possibility that genistein forms through hydrolytic decomposition of the storage form and it implies that the defence mechanisms in G. tinctoria suspension can follow various courses depending directly on the type of elicitor.
The maximum concentration of genistein and daidzein when chitosan was used as elicitor was, respectively, 24% and 13% higher than the concentrations of these compounds obtained when the cultures were supplemented with methyl jasmonate (Tables 2 and 3). Moreover, since the production profile of genistin malonate was maintained (max. concentration: 73.6 mg/100 g DW, Table 3), G. tinctoria biomass elicited with chitosan constitutes biological material with a generally higher content of isoflavones than the biomass supplemented with methyl jasmonate (Tables 2 and 3). Therefore, this culture is more attractive technologically.
Irrespective of the moment when it was added to the medium, chitosan did not play the role of a permeabilising factor in G. tinctoria suspension. In the investigated culture, all of the analysed secondary metabolites were stored intracellularly, similarly to the basic biomass and the suspension elicited with methyl jasmonate.
The effect of a DMSO supplement on the growth of G. tinctoria suspension and the accumulation of isoflavonoids
Unlike physical permeabilisation, chemical permeabilisation is a relatively inexpensive and simple method allowing for a release of secondary metabolites from plant matrices to growth media (Bordelius 1988; Kanaraiah et al. 2005; Knorr and Berlin 1987; Komolpis et al. 1998; Teissie et al. 2005). The choice of DMSO to permeabilise G. tinctoria suspension was based on literature reports indicating that this compound is one of the most effective factors changing cell permeability and causing not only a considerable release of the analysed metabolites into the growth media, but also, in low concentration (2–10%) allowing the cultivated tissues to remain biochemically active (Bordelius 1988; Knorr and Berlin 1987; Komolpis et al. 1998; Teissie et al. 2005).
In order to avoid decay of G. tinctoria biomass when cultivated in the presence of DMSO (Bordelius 1988; Knorr and Berlin 1987), the compound was added to the medium in relatively low concentrations (20 ml l−1), and only on day 26 of the experiment, i.e. after the intensive growth of cells was completed and the maximum concentration of isoflavones in the culture was achieved (Table 4 and Fig. 5).
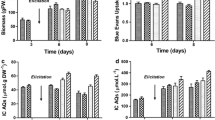
It was observed that in G. tinctoria suspension DMSO caused a release of isoflavones into the growth medium, causing at the same time gradual decay of the cells in the culture (Tables 4, 5 and Figs. 5, 6, 7, 8). Unlike the basic culture, G. tinctoria suspension cultivated in the presence of DMSO decayed relatively quickly. The amount of DW (28.6 g l−1) and FW (532.3 g l−1) obtained in the last hour of the experiment were lower by 23 and 28%, respectively, than in the control suspension (Fig. 5).
The 2% concentration of DMSO in G. tinctoria suspension proved sufficient to change the permeability of the investigated cells. In the described conditions gradual release of all isoflavonoids synthesised in the biomass into the growth medium was obtained. The process, however, was quite lengthy (Figs. 6, 7, 8). Contrary to literature reports where maximum concentrations of the analysed metabolites were observed in media already half an hour after permeabilisation (Bordelius 1988; Knorr and Berlin 1987), in the described experiment this result was only achieved in the 7th hour of the experiment (Table 5 and Figs. 6, 7, 8). The relatively slow release of isoflavonoids from G. tinctoria cells was probably caused by low concentration of DMSO in the culture (2%) and the fact that it was only supplemented in the stationary phase of biomass growth, i.e. prior to the adoption of the experimental procedure which was different from the previously described models (Bordelius 1988; Gontier et al. 2002; Knorr and Berlin 1987; Komolpis et al. 1998).
Analysing the concentration of isoflavones in the media and plant matrices, including aglycones (genistein and daidzein), glycosides (genistin, genistein 7-O-diglucoside, 2′-hydroxygenistein 7-O-glucoside, daidzin) and esters (genistin malonate and genistin acetate), it was found out that both the grade and the speed of the individual compound’s leakage from the cells are directly related to the chemical structure of these isoflavones (Table 5). In view of the above, the first compounds to be found in the growth medium were esters and diglycosides (30 min after permeabilisation) and later glycosides and aglycones (60, 90 and 120 min after supplementing with DMSO, respectively—Table 5). The results indicate that the rate at which isoflavones are released from G. tinctoria plant matrix is in direct proportion to the polarity of the investigated compounds. The polarity also determines the degree to which isoflavones are released from the biomass, i.e. the total concentration of the investigated compounds in the experimental media. Generally speaking, for isoflavonoid esters it was approximately 95% of the total concentration of these compounds accumulated in G. tinctoria suspension on day 26 of the experiment (Fig. 8) and for glycosides and aglycones 80 and 67%, respectively, was released into the growth medium (Figs. 6 and 7).
High concentration of isoflavones in the experimental media corresponds to the results achieved when in vitro plant cultures were permeabilised with DMSO at 25% concentration (Bordelius 1988). The achieved result indicates that G. tinctoria biomass is exceptionally susceptible to permeabilisation with this compound and does not require high concentrations of DMSO which could considerably reduce cell metabolic activity (Bordelius 1988; Gontier et al. 2002).
Irrespective of the structure of the investigated isoflavones, maximum concentration of all analysed compounds was achieved in the media in the 7th hour of the experiment, and a continued experiment did not lead to any further release of the accumulated metabolites into the media (Figs. 6, 7, 8).
Conclusions
The controlled growth process with elicitation and permeabilisation of G. tinctoria suspension led to the achievement of the main objective of the experiment, which was to obtain a plant in vitro system producing more phytoestrogens than the basic culture, with extracellular storage profile.
The series of experiments using chitosan and methyl jasmonate confirmed the key role of stress factors in the biosynthesis of phytoestrogenic isoflavones. It was also shown that the type of elicitor has an impact on the formation mechanism of various phytoalexins in G. tinctoria suspension. The appropriate moment for adding the stress factor to the growth system is related to the point of highest concentration of the isoflavonoid compounds in the biomass, which is essential when treating an in vitro culture as a source of particular secondary metabolites. The achieved results indicate that the broadly understood elicitation of G. tinctoria suspension should be the direction to be followed in further research into controlled biosynthesis of isoflavones in in vitro cultures of this species with the objective of developing a highly productive biotechnological system to produce medically active isoflavones. Moreover, given its rich isoflavonoid fraction and considerable susceptibility to elicitation, G. tinctoria biomass may be used in the future as a model system for basic experiments into the relations between plants and their environment.
Permeabilisation of G. tinctoria suspension with 2% DMSO proved that in the investigated growth system it is possible to achieve a desired release of isoflavones into the experimental media in concentrations which justify using this procedure in a technological process. It has to be noted, however, that it is only appropriate for a periodic culture. The modification of the growth conditions developed here for the purposes of a continuous plant in vitro system will require a series of further experiments to develop the way of maintaining vitality of G. tinctoria biomass while cultivating the suspension with the permeabilising factor.
References
Armero J, Requejo R, Jorrin J, Lòpez-Valbuena R, Tena M (2001) Release of phytoalexins and related isolavonoids from intact chickpea seedlings elicited with reduced glutathione at root level. Plant Physiol Biochem 39:785–795
Barz W, Mackenbrock U (1994) Constitutive and elicitation induced metabolism of isoflavones and pterocarpans in chickpea (Cicer arietinum) cell suspension cultures. Plant Cell Tiss Org Cult 38:199–211
Bednarek P, Frański R, Kerhoas L, Einhorn J, Wojtaszek P, Stobiecki M (2001) Profiling changes in metabolism of isoflavonoids and their conjugates in Lupinus albus treated with biotic elicitor. Phytochemistry 56:77–85
Bhuiyan MdNH, Adachi T (2003) Stimulation of betacyanin synthesis through exogenous methyl jasmonate and other elicitors in suspension-culture cells of Portulaca. J Plant Physiol 160:1117–1124
Bordelius P (1988) Permeabilization of plant cells for release of intracellularly stored products: viability studies. Appl Microbiol Biotechnol 27:561–566
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Brandi ML (1997) Natural and synthetic isoflavones in the prevention of treatment of chronic diseases. Calcif Tissue Int 61:55–58
Chang-He Z, Hui-Bi X (2001) Improved paclitaxel production by in situ extraction and elicitation in cell suspension cultures of Taxus chinensis. Biotechnol Lett 23:189–193
Dixon RA, Ferreira D (2002) Molecules of interest, genistein. Phytochemistry 60:205–211
Ebdel J, Mithöfer A (1998) Early events in the elicitation of plant defense. Planta 206:335–348
Edwards R, Daniell TJ, Gregory ACE (1997) Methylation reactions and the phytoalexin response in alfalfa suspension cultures. Planta 201:359–367
Gagnon H, Ibrahim RK (1997) Effects of various elicitors on the accumulation and secretion of isoflavonoids in white lupin. Phytochemistry 44:1463–1467
Gontier E, Clement A, Tran TLM, Gravot A, Lievre K, Guckert A, Bourgaud F (2002) Hydroponic combined with natural or forced root permeabilization: a promising technique for plant secondary metabolite production. Plant Sci 163:723–732
Haberder H, Schröder G, Ebel J (1989) Rapid induction of phenylalanine ammonia-lyase and chalcone synthase mRNAs during fungus infection of soybean (Glycine max L.) roots or elicitor treatment of soybean cell cultures at the onset of phytoalexin synthesis. Planta 177:58–65
Kanaraiah P, Kavi Kishor PB, Carlsson M, Magnusson KE, Mandenins CF (2005) Enhancement of anthraquinone accumulation in Morinda citrifolia suspension cultures. Plant Sci 168:1337–1344
Knorr D, Berlin J (1987) Effects of immobilization and permeabilization procedures on growth of Chenopodium rubrum and amaranthin concentration. J Food Sci 52:1397–1400
Komaraiah P, Naga Amrutha R, Kavi Kishor PB, Ramakrishna SV (2002) Elicitor enhanced production of plumbagin in suspension cultures of Plumbago rosea L. Enz Microbiol Technol 31:634–639
Komolpis K, Kaufman PB, Wang HY (1998) Chemical permeabilization and in situ removal of daidzein from biologically viable soybean (Glycine max) seeds. Biotechnol Techn 12:697–700
Lozovaya VV, Lygin AV, Zernova OV, Li S, Hartman GL, Widholm JM (2004) Isoflavonoid accumulation in soybean hairy roots upon treatment with Fusarium solani. Plant Physiol Biochem 42:671–679
Luczkiewicz M, Głód D (2003) Callus cultures of Genista plants-in vitro material producing high amounts of isoflavones of phytoestrogenic activity. Plant Sci 165:1101–1108
Luczkiewicz M, Głód D (2005) Morphogenesis-dependent accumulation of phytoestrogens in Genista tinctoria in vitro cultures. Plant Sci 168:967–979
Luczkiewicz M, Głód D, Bączek T, Buciński A (2004) LC-DAD UV and LC-MS for the analysis of isoflavones and flavones from in vitro and in vivo biomass of Genista tinctoria L. Chromatographia 60:179–185
Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41:391–398
Paiva NL, Oommen A, Harrison MJ, Dixon RA (1994) Regulation of isoflavonoid metabolism in alfalfa. Plant Cell Tiss Org Cult 38:213–220
Park HH, Hakamatsuka T, Sankawa U, Ebizuka Y (1995) Rapid metabolism of isoflavonoids in elicitor-treated cell suspension cultures of Pueraria lobata. Phytochemistry 38:373–380
Pitta-Alvarez SI, Spollansky TC, Giulietti AM (2000) The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enz Microb Technol 26:252–258
Radzikowski Cz, Wietrzyk J, Grynkiewicz G, Opolski A (2004) Genistein: a soy isoflavone revealing a pleiotropic mechanism of action-clinical implications in the treatment and prevention of cancer. Postepy Hig Med Dosw 58:128–139
Ramachandra Rao S, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204
Schenk P, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinate plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97:11655–11660
Staniszewska I, Królicka A, Malińska E, Wojkowska E, Szafranek J (2003) Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enz Microbiol Technol 33:565–568
Teissie J, Golzio M, Rols MP (2005) Mechanism of cell membrane electropermeabilization: a minireview of our present (lack of ?) knowledge. Biochim Biophys Acta 1724:270–280
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Lewak.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Luczkiewicz, M., Kokotkiewicz, A. Elicitation and permeabilisation affect the accumulation and storage profile of phytoestrogens in high productive suspension cultures of Genista tinctoria . Acta Physiol Plant 34, 1–16 (2012). https://doi.org/10.1007/s11738-011-0799-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0799-4