Abstract
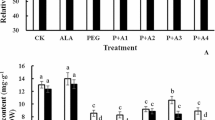
The effect of sodium nitroprusside (SNP; nitric oxide donor) treatment on wheat plant (Triticum aestivum L.) under drought stress during grain filling stage was investigated. When two cultivars wheat plants, Yumai No. 949 and Shanmai No. 5, were drought stressed by PEG for 72 h and rewatered for 48 h, the affections of osmotic dehydration and rehydration on the antioxidant enzymes activities and psbA gene transcriptional abundance were compared. Relative water contents (RWC) decreased markedly after 72 h of PEG stress, along with an obvious decrease in chlorophyll content, increase in SOD, CAT and APX activities, and MDA content as well. Real-time quantitative polymerase chain amplification indicated that drought stress also remarkably inhibited the transcription of psbA gene in photosystem II (PSII). All of these responses could be restored by removing of stress and applying another 48 h of rewatering. The exogenous 0.2 mmol l−1 SNP treatment could significantly alleviate the stress injury and accelerate the progress of recovery. Compared to Yumai No. 949, Shanmai No. 5 had less destroyed plasma membranes, higher RWC and chlorophyll contents, more psbA gene transcriptional abundance during water stress, and rapider recovery to control after rewatering, suggesting not only a better drought resistance but also a better recovery capability after a severe drought stress. The present results also suggested that the application of exogenous SNP could enhance the stress resistance of wheat plant during grain filling stage by increasing antioxidant enzymes activities, as well as protecting important gene transcription in PSII, which were to the benefit of functional recovery from drought stress.




Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalases
- DPS:
-
Data processing system
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- PEG:
-
Polyethylene glycol
- PPFD:
-
Photosynthetic photon flux density
- PSII:
-
Photosystem II
- PVP:
-
Polyvinylpyrrolidone
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SNP:
-
Sodium nitroprusside
- SOD:
-
Superoxide dismutase
References
Beligni MV, Lamattina L (1999) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208:337–344
Böhm FMLZ, Ferrarese MdLL, Zanardo DIL, Magalhaes JR, Ferrarese-Filho O (2010) Nitric oxide affecting root growth, lignification and related enzymes in soybean seedlings. Acta Physiol Plant 32:1039–1046
Bray E (1993) Molecular responses to water deficit. Plant Physiol 103:1035
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Conner EM, Grisham MB (1996) Inflammation, free radicals, and antioxidants. Nutrition 12:274–277
Duan HG, Yuan S, Liu WJ, Xi DH, Qing DH, Lin HH (2006) Effects of exogenous spermidine on photosystem II of wheat seedlings under water stress. J Integr Plant Biol 48:920–927
Edelman M, Mattoo AK (2008) D1-protein dynamics in photosystem II: the lingering enigma. Photosynth Res 98:609–620
Frank S, Kampfer H, Podda M, Kaufmann R, Pfeilschifter J (2000) Identification of copper/zinc superoxide dismutase as a nitric oxideregulated gene in human (HaCaT) keratinocytes: implications for keratinocyte proliferation. Biochem J 346:719–728
He JX, Wen JQ, Chong K, Liang HG (1998) Changes in transcript levels of chloroplast psbA and psbD genes during water stress in wheat levels. Physiol Plantarum 102:49–54
He J, An L, Lin H, Liang H (1999) Evidence for transcriptional and post-transcriptional control of protein synthesis in water-stressed wheat leaves: a quantitative analysis of messenger and ribosomal RNA. J Plant Physiol 155:63–69
Hsiao T (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570
Khanna-Chopra R, Selote DS (2007) Acclimation to drought stress generates oxidative stress tolerance in drought resistant than susceptible wheat cultivar under field conditions. Environ Exp Bot 60:276–283
Leucci MR, Lenucci MS, Piro G, Dalessandro G (2008) Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J Plant Physiol 165:1168–1180
Liu WJ, Yuan S, Zhang NH, Lei T, Duan HG, Liang HG, Lin HH (2006) Effect of water stress on photosystem 2 in two wheat cultivars. Biol Plantarum 50:597–602
Liu Y, Xu S, Ling T, Xu L, Shen W (2010) Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J Plant Physiol 167:1371–1379
Madani A, Rad AS, Pazoki A, Nourmohammadi G, Zarghami R (2010) Wheat (Triticum aestivum L.) grain filling and dry matter partitioning responses to source:sink modifications under postanthesis water and nitrogen deficiency. Acta Sci Agron 32:145–151
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mulo P, Sicora C, Aro EM (2009) Cyanobacterial psbA gene family: optimization of oxygenic photosynthesis. Cell Mol Life Sci 66:3697–3710
Pandey DM, Yeo U-D (2008) Stress-induced degradation of D1 protein and its photoprotection by DCPIP in isolated thylakoid membranes of barley leaf. Biol Plantarum 52:291–298
Phan C, Letouze R (1983) A comparative study of chlorophyll, phenolic and protein contents, and of hydroxycinnamate: CoA ligase activity of normal and vitreous’ plants (Prunus avium L.) obtained in vitro. Plant Sci Lett 31:323–327
Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Qian H, Sheng G, Liu W, Lu Y, Liu Z, Fu Z (2008) Inhibitory effects of atrazine on Chlorella vulgaris as assessed by real time polymerase chain reaction. Environ Toxicol Chem 27:182–187
Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C (2006) Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ 29:2143–2152
Sairam R, Srivastava G (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Sairam RK, Deshmukh PS, Saxena DC (1998) Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol Plant 41:387–394
Sangtarash MH (2010) Responses of different wheat genotypes to drought stress applied at different growth stages. Pak J Biol Sci 13:114–119
Selote DS, Khanna-Chopra R (2010) Antioxidant response of wheat roots to drought acclimation. Protoplasma 245:153–163
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Sharma PK, Singhal GS (1993) Effect of water stress on primary photosynthetic processes: interaction with light and temperature. Indian J Biochem Biophys 30:10–14
Shu Y, Hong-Hui L (2004) Transcription, translation, degradation, and circadian clock. Biochem Biophys Res Commun 321:1–6
Simova-Stoilova L, Demirevska K, Petrova T, Tsenov N, Feller U (2009) Antioxidative protection and proteolytic activity in tolerant and sensitive wheat (Triticum aestivum L.) varieties subjected to long-term field drought. Plant Growth Regul 58:107–117
Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2:503
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Tian X, Lei Y (2006) Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plantarum 50:775–778
Wang YX, Sun GR, Suo B, Chen G, Wang JB, Yan Y (2008) Effects of Na2CO3 and NaCl stresses on the antioxidant enzymes of chloroplasts and chlorophyll fluorescence parameters of leaves of Puccinellia tenuiflora (Turcz.) scribn.et Merr. Acta Physiol Plant 30:143–150
Wang G-P, Hui Z, Li F, Zhao M-R, Zhang J, Wang W (2010) Improvement of heat and drought photosynthetic tolerance in wheat by overaccumulation of glycinebetaine. Plant Biotechnol Rep 4:213–222
Xuan W, Huang L, Li M, Huang B, Xu S, Liu H, Gao Y, Shen W (2007) Induction of growth elongation in wheat root segments by heme molecules: a regulatory role of carbon monoxide in plants? Plant Growth Regul 52:41–51
Yamamoto Y (2001) Quality control of photosystem II. Plant Cell Physiol 42:121–128
Yang J, Zhang J, Huang Z, Zhu Q, Wang L (2000) Remobilization of carbon reserves is improved by controlled soil drying during grain filling of wheat. Crop Sci 40:1645–1655
Yuan S, Liu WJ, Zhang NH, Wang MB, Liang HG, Lin HH (2005) Effects of water stress on major photosystem II gene expression and protein metabolism in barley leaves. Physiol Plantarum 125:464–473
Acknowledgments
This study is supported by National Natural Science Foundation of China (No. 31000688 and No. 30971725).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Weidner.
Rights and permissions
About this article
Cite this article
Wang, Y., Suo, B., Zhao, T. et al. Effect of nitric oxide treatment on antioxidant responses and psbA gene expression in two wheat cultivars during grain filling stage under drought stress and rewatering. Acta Physiol Plant 33, 1923–1932 (2011). https://doi.org/10.1007/s11738-011-0740-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0740-x