Abstract
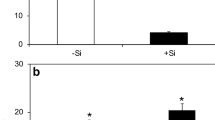
Silicon (Si) has been verified to play an important role in enhancing plant resistance against pathogens, but the exact mechanisms remain unclear. Two near-isogenic lines of rice (Oryza sativa L.), CO39 (blast susceptible), and C101LAC (Pi-1) (blast resistant), were hydroponically grown to study the effects of exogenous silicon application on the changes of disease incidence, mineral nutrient concentrations, chlorophyll content, and photochemical efficiency in Magnaporthe oryzae infected rice plants. Si amendment in nutrient solution at a concentration of 2.0 mM significantly reduced the disease index of rice plants of CO39 and C101LAC (Pi-1). Silicon application alone had no effects on mineral nutrient contents, chlorophyll content, maximum/potential quantum efficiency (F v/F m), and the maximum primary yield (F v/F 0) of photochemistry of PS II in healthy rice leaves. M. oryzae inoculation significantly increased the content of K, Na, Ca, Mg, Fe, and reduced the value of F v/F 0 and F v/F m in rice leaves. However, Si treatment suppressed M. oryzae induced increase of mineral nutrient contents, and significantly increased F v/F 0 and F v/F m value compared with Si-deficient infected plants. These results suggest that silicon-enhanced resistance to rice blast is associated with an enhancement of photochemical efficiency and adjustment of mineral nutrient absorption in M. oryzae-infected rice plants.




Similar content being viewed by others
References
Araus JL, Amaro Voltas TJ, Nakkoul H, Nachit MM (1998) Chlorophyll fluorescence as a selection criterion for grain yield in durum wheat under Mediterranean conditions. Field Crops Res 55:209–223
Awad AS, Edward DG, Campbell LC (1990) Phosphorus enhancement of salt tolerance of tomato. Crop Sci 30:123–128
Bains SS, Jhooty JS (1984) The relationship between cation-ratio and host-resistance to certain downy mildew and root-knot diseases. Plant Soil 81:69–74
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Balasubramanian KA (1973) Influence of nitrogen and phosphorus fertilizers on the expression of downy mildew of sorghum. Plant Soil 38:621–626
Bassanezi RB, Amorim L, Filho AB, Berger RD (2002) Gas exchange and emission of chlorophyll fluorescence during the monocycle of rust, angular leaf spot and anthracnose on bean leaves as a function of their trophic characteristics. J Phytopathol 150:37–47
Bastiaans L (1991) Ratio between virtual and visual lesion size as a measure to describe reduction in leaf photosynthesis of rice due to leaf blast. Phytopathology 81:611–615
Bastiaans L, Roumen EC (1993) Effect on leaf photosynthetic rate by leaf blast for rice cultivars with different types and levels of resistance. Euphytica 66:81–87
Berger S, Papadopoulos M, Schreiber U, Kaiser W, Roitsch T (2004) Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol Plant 122:419–428
Bonfig KB, Schreiber U, Gabler A, Roitsch T, Berge S (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225:1–12
Bowen P, Menzies J, Ehret D, Samuels L, Glass ADM (1992) Soluble silicon sprays inhibit powdery mildew development on grape leaves. J Am Soc Hortic Sci 117:906–912
Cai KZ, Gao D, Luo SM, Zeng RS, Yang JY, Zhu XY (2008) Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol Plant 134:324–333
Cai KZ, Gao D, Chen JN, Luo SM (2009) Probing the mechanisms of silicon-mediated pathogen resistance. Plant Signal Behav 4:1–3
Chia TF, He J (1999) Photosynthesic capacity in Oncidium (Orchidacceae) plants after virus eradication. Environ Exp Bot 42:11–16
Chou H, Bundock N, Rolfe SA, Scholes JD (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol Plant Pathol 1:99–113
Côté-Beaulieu C, Chain F, Menzies JG, Kinrade SD, Bélanger RR (2009) Absorption of aqueous inorganic and organic silicon compounds by wheat and their effect on growth and powdery mildew control. Environ Exp Bot 65:155–161
Datnoff LE, Deren CW, Snyder GH (1997) Silicon fertilization for disease management of rice in Florida. Crop Prot 16:525–531
Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol Lett 249:1–6
Fauteux F, Chain F, Belzile F, Menzies J, Bélanger RR (2006) The protective role silicon in the Arabidopsis-powdery mildew pathosystem. PNAS 103:17554–17559
Fawe A, Abou-Zaid M, Menzies JG, Bélanger RR (1998) Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 88:396–401
Grieve CM, Fujiyama H (1987) The response of two cultivars to external Na/Ca ratio. Plant Soil 103:245–250
Guo DP, Zhao JP, Liu H, Peng Y, Wang QM, Chen JS, Rao GZ (2005) Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var tsatsai) after turnip mosaic virus infection. Plant Sci 168:57–63
Hayasaka T, Fujii H, Ishiguro K (2008) The role of silicon in preventing appressorial penetration by the rice blast fungus. Phytopathology 98:1038–1044
Heath MC, Stumpf MA (1986) Ultrastructural observations of penetration sites of the cowpea rust fungus in untreated and silicon-depleted French bean cells. Physiol Mol Plant Pathol 29:27–39
IRRI (2002) Standard evaluation system for rice (SES). Los Baños, Philippines, 56 pp
Keeping MG, Meyer JH (2002) Calcium silicate enhances resistance of sugarcane to the African stalk borer Eldana saccharina Walker (Lepidoptera: Pyralidae). Agric For Entomol 4:265–274
Kim SG, Kim KW, Park EW, Choi D (2002) Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathology 92:1095–1103
Lanning FC, Eleuterius LN (1989) Silica deposition in some C3 and C4 species of grasses, sedges and composites in the USA. Ann Bot 64:395–410
Lee GJ, Duncan RR, Carrow RN (2007) Nutrient uptake responses and inorganic ion contribution to solute potential under salinity stress in halophytic seashore Paspalums. Crop Sci 47:2504–2512
Liang YC, Sun WC, Zhu YG, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Lichtenthaler HK, Miehé JA (1997) Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci 2:316–320
Ma JF, Takahashi E (1993) Interaction between calcium and silicon in water-cultured rice plants. Plant Soil 148:107–113
Menzies JG, Ehret DL, Glass ADM, Helmer T, Koch C, Seywerd F (1991) Effects of soluble silicon on the parasitic fitness of Sphaerotheca fuliginea on Cucumis sativus. Phytopathology 81:84–88
Ogren E, Evans JR (1992) Photoinhibition in situ in six species of Eucalyptus. Aust J Plant Physiol 19:223–232
Parry DW, Smithson F (1964) Types of opaline silica deposition in the leaves of British grasses. Ann Bot 23:169–185
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Rahoutei J, García-Luque I, Barón M (2000) Inhibition of photosynthesis by viral infection: effect on PSII structure and function. Physiol Plant 110:286–292
Rémus-Borel W, Menzies JG, Bélanger RR (2005) Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol Mol Plant Pathol 66:108–115
Ribot C, Hirsch J, Balzergue S, Tharreau D, Notteghem JL, Lebrun MH, Morel JB (2008) Susceptibility of rice to the blast fungus Magnaporthe grisea. J Plant Physiol 165:114–124
Rodrigues FÁ, Vale FXR, Korndörfer GH, Prabhu AS, Datnoff LE, Oliveira AMA, Zambolim L (2003) Influence of silicon on sheath blight of rice in Brazil. Crop Prot 22:23–29
Rodrigues FÁ, McNally DJ, Datnoff LE, Jones JB, Labbé C, Benhamou N, Menzies JG, Bélanger RR (2004) Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathology 94:177–183
Samuels Al, Glass ADM, Ehret DL, Menzies JG (1991) Mobility and deposition of silicon in cucumber plants. Plant Cell Environ 14:485–492
Schnabel G, Strittmatter G, Noga G (1998) Changes in photosynthetic electron transport in potato cultivars with different field resistance after infection with Phytophthora infestans. J Phytopathol 146:205–210
Seebold KW, Kucharek TA, Datnoff LE, Correa-victoria FJ, Marchetti MA (2001) The influence of silicon on components of resistance to blast in susceptible, partially resistant and resistant cultivars of rice. Phytopathology 91:63–69
Shangguan ZP, Shao MA, Dyckmans J (2000) Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J Plant Physiol 156:46–51
Swiech R, Browning S, Molsen D, Stenger DC, Holbrook GP (2001) Photosynthetic responses of sugar beet and Nicotiana benthamiana Domin infected with beet curly topvirus. Physiol Mol Plant Pathol 58:43–52
Van der Vorm PDJ (1987) Dry ashing of plant material and dissolution of the ash in HF for the colorimetric determination of silicon. Commun Soil Sci Plant Anal 18:1181–1189
Van Kooten O, Meurs C, Van Loon LC (1990) Photosynthetic electron transport in tobacco leaves infected with tobacco mosaic virus. Physiol Plant 80:446–452
Walters DR, Bingham IJ (2007) Influence of nutrition on disease development caused by fungal pathogens: implications for plant disease control. Ann Appl Biol 3:307–324
Wang XS, Han JG (2007) Effects of NaCl and silicon on ion distribution in the roots, shoots and leaves of two alfalfa cultivars with different salt tolerance. Soil Sci Plant Nutr 53:278–285
Watanabe S, Shimoi E, Ohkama N, Hayashi H, Yoneyama T, Yazaki J, Fujii F, Shinbo K, Yamamoto K, Sakata K, Sasaki T, Kishimoto N, Kikuchi S, Fujiwara T (2004) Identification of several rice genes regulated by Si nutrition. Soil Sci Plant Nutr 50:1273–1276
Wolfe MS (2000) Crop strength through diversity. Nature 406:681–682
Zhu YY, Chen HR, Fan JH, Wang YY, Li Y, Chen JB, Fan JX, Yang SS, Hu LP, Leungk H, Mewk TW, Tengk PS, Wang ZH, Mundtk CC (2000) Genetic diversity and disease control in rice. Nature 406:718–722
Acknowledgments
This study was financially supported by grants from the National Key Basic Research Funds of China (2006CB1002006), Natural Science Foundation (31070396) and the earmarked fund for Modern Agro-industry Technology Research System.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Barna.
Rights and permissions
About this article
Cite this article
Gao, D., Cai, K., Chen, J. et al. Silicon enhances photochemical efficiency and adjusts mineral nutrient absorption in Magnaporthe oryzae infected rice plants. Acta Physiol Plant 33, 675–682 (2011). https://doi.org/10.1007/s11738-010-0588-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0588-5