Abstract
The aim of the research was to estimate the sensitivity of tomato tissue and spore from necrotrophic isolate of B. cinerea on H2O2. The influence of exogenic H2O2 and B. cinerea on plant tissue and on the activity of peroxidases (PO), catalase (CAT) and superoxide dismutase (SOD) in apoplastic tomato leaves fraction were investigated.
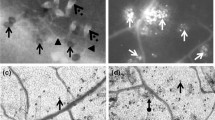
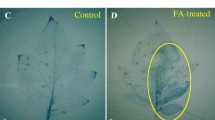
It was proved that 40 mM H2O2 damaged the cells of a host, and inhibited in vitro germination of B.cinerea spores. Complete inhibition of germination was observed after the use 100 mM H2O2. In the presence of spores H2O2 was decomposed to H2O and O2. Trace activity of catalase was observed in a solution of spores used for inoculation. Necrosis which appeared on the leaves after 40 mM H2O2 treatment resembled hypersensitive response. On the leaves pretreated at this concentration the development of infection was observed. The H2O2 concentration harmful for the tissues, stimulated the PO activity measured with NADH — responsible for generation of ·O −2 , as well as with syringaldazine (S) and ferulic acid (FA), substrates characteristics of forms lignifying and strengthening the cell wall. Clear increase in CAT activity, resulting from infection and early pretreatment with H2O2 was observed in apoplast. No effect on SOD activity was observed.
A hypothesis may be put forward, that germinating spores produce enzymes which allow them to decompose H2O2 generated in apoplast, so there is little likelihood that B. cinerea can be directly inhibited by reactive oxygen forms (ROS) during initial stages of infection. Necrotic lesions resembling HR generated by exogenous H2O2 as well as induction of activity of apoplastic plant enzymes, particularly PO connected with strengthening and lignification of cell wall, were not sufficient factors to inhibit fungal expansion.
Similar content being viewed by others
Abbreviations
- ROS:
-
reactive oxygen species
- CAT:
-
catalase
- PO:
-
peroxidase
- SOD:
-
superoxide dismutase
- TMB:
-
tetramethylbenzidine
- FA:
-
ferulic acid
- S:
-
syringaldazine
- MBTH:
-
3-methylbenzothiazoline hydrazone
References
Apostol I., Heinstein P.F., Low P.S. 1989. Rapid stimulation an oxidative burst during elicitation of cultured plant cells. Role in defence and signal transduction. Plant Physiol. 90: 109–116.
Baker C.J., Orlandi E.W. 1995. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33: 299–321.
Barcelo R.A. 1998. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH oxidase-like enzyme. Planta 207: 207–216.
Bestwick C.S., Brown I.R., Mansfield J.W. 1998. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol. 118: 1067–1078.
Beauchamp C., Fridovich I. 1971. Superoxide dismutase improved assays and assay applicable to acrylamide gels. Anal. Biochem. 44: 276–287.
Bowell G.P., Davies D.R., Gerrish C., Auh C-K., Murphy T.M. 1998. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 116: 1379–1385.
Bradford M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Bradley D.J., Kjellbom P., Lamb C.J. 1992. Elicitor-and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70: 21–30.
Capaldi D.J., Taylor K.E. 1983. A new peroxidase color reaction: oxidative coupling of 3-methyl-2-benzothiazolinone hydrazone (MBTH) with its formaldehyde azine application to glucose and choline oxidases. Anal. Biochem. 129: 329–336.
Creissen G., Firmin J., Freyer M., Kular B., Leyland N., Reynolds H., Pastori G., Wellburn F., Baker N., Wellburn A., Mullineaux P. 1999. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11: 1277–1292.
Doke N. 1983. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophtora infestans and to the hyphal wall components. Physiol. Plant Pathol. 23: 345–357.
Dhindsa R.S., Plumb-Dhindsa P., Thorpe T.A. 1981. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decrease levels of superoxide dismutase and catalase. J. Exp. Bot. 32: 93–101.
Durrant W.E., Rowland O., Piedras P., Hammond-Kosack K.E., Jones J.D.G. 2000. cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell 12: 963–977.
Figueroa-Spinoza M.C., Rouau X. 1998. Oxidative cross-linking of pentosans by a fungal laccase and horseradish peroxidase: Mechanism of linking between feruloylated arabinoxylans. Cereal Chem. 75: 259–265.
Frahry G., Schopfer P. 1998. Hydrogen peroxide production by roots and its stimulation by exogenous NADH. Physiol. Plantarum 103: 395–404.
Fry S.C. 1998. Oxidative scission of plant cell wall polysacchardes by ascorbate-induced hydroxy radicals. Biochem. J. 332: 507–515.
Gil-ad N.L., Bar-Nun N., Noy T., Mayer A.M. 2000. Enzymes of Botrytis cinerea capable of breaking down hydrogen peroxide. FEMS Microb. Letters 190: 121–126.
Govrin E.M., Levine A. 2000. The hypersensitive response facilitates plant infection by the nectrotrophic pathogen Botrytis cinerea. Current Biology 10: 751–757.
Halliwell B., Guttering J.M.C. 1990. Role of free radicals and catalytic metal ions in human disease: An overview. Methods in Enzymology 186: 1–85.
Hckelhoven R., Kogel K-H. 2003. Reactive oxygen intermediates in plant-microbe interactions: Who is who in powdery mildew resistance? Planta 216: 891–902.
Imberty A., Goldberg R., Catesson A.M. 1985. Isolation and characterization of Populus isoperoxidases involved in the last step of lignification. Planta 164: 221–226.
Ishida A., Ookubu K., Ono K. 1987. Formation of hydrogen peroxide by NAD(P)H oxidation with isolated cell wall-associated peroxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol. 28(4): 723–726.
Lamb C.J., Brisson L., Bradley D.J., Kjellborn P. 1993. Stimulus-dependent oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defence response and control point in cellular maturation. In: Friting B, Legrand M, ed. Mechanisms of Plant Defence Responses. Kluwar Academic Publisher, 250–256.
Lamb C., Dixon R.A. 1997. The oxidative burst in plant disease resistance. Annual Rev. Plant Physiol. Mol. Biol. 48: 251–275.
Levine A., Tenhaken R., Dixon R., Lamb C. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593.
Lu H., Higgins V.J. 1999. The effect of hydrogen peroxide on the viability of tomato cells and of the fungal pathogen Cladosporium fulvum. Physiol. Mol. Plant Pathol. 54: 131–143.
Lüthje S., Böttger M., Döring O. 2000. Are plants stacked neutrophils? Comparison of pathogen-induced oxidative burst in plants and mammals. Progress in Botany 61: 187–222.
Malolepsza U., Urbanek H. 2000. The oxidants and antioxidant enzymes in tomato leaves treated with o-hydroxyethylorutin and infected with Botrytis cinerea. Eur. J. Plant Pathol. 106: 657–665.
Mayer A. M., Staples R.C., Gil-ad N.L. 2001. Mechanisms of survival fungal plant pathogens in hosts expressing the hypersensitive response. Phytochemistry 58: 33–41.
Minibayeva F.V., Gordon L.K., Kolesnikov O.P., Chasov A.V. 2001. Role of extracellular peroxidase in the superoxide production by wheat root cells. Protoplasma 217: 125–128.
Milosevic N., Slusarenko A.J. 1996. Activity oxygen metabolism and lignification in the hypersensitive response in bean. Physiol. Mol. Plant Pathol. 49: 143–158.
Moerschbacher B.M., Noll U., Gorrichon I., Reisener H-J. 1990. Specific inhibition of lignification breaks hypersensitive resistance to wheat stem rust. Plant Physiology 93: 465–470.
Ogawa K., Kanematsu S., Asada K. 1997. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol. 38: 1118–1126.
Patykowski J., Urbanek H. 2003. Activity of enzymes related to H2O2 generation and metabolism in leaf apoplastic fraction of tomato leaves infected with Botrytis cinerea. J. Phytopathology 151: 153–161.
Peng M., Ku J. 1992. Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and tobacco leaf discs. Phytopathology 64: 696–699.
Rij R.E., Forney C.F. 1995. Phytotoxicity of vapour phase hydrogen peroxide to Thompson Seedlees grapes and Botrytis cinerea spores. Crop Protection 14: 131–135.
Schouten A., Tenberge K.B., Vermeer J., Stewart J., Wagemaker L., Williamson B., van Kan J.A.L. 2002. Functional analysis of an extracellular catalase of Botrytis cinerea. Mol. Plant Pathol. 3: 227–238.
Schweikert C., Liszkay A., Schopfer P. 2000. Scission of polysaccharide by peroxidase-generated hydroxyl radicals. Phytochemistry 53: 565–570.
Takahama U. 1995. Oxidation of hydroxycinnamic acid and hydroxycinnamyl alcohol derivatives by laccase and peroxidase. Interactions among p-hydroxyphenyl, guaiacyl and syringyl groups during the oxidation reactions. Physiol. Plant. 93: 61–68.
Tiedemann A.v. 1997. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol. Mol. Plant Pathol. 50: 151–166.
Wojtaszek P. 1997. Oxidative burst: an early plant response to pathogen infection. Biochem. J. 322: 681–692.
Zimmerlin A., Wojtaszek P., Bolwell G.P. 1994. Synthesis of dehydrogenation polymers of ferulic acid with high specificity by a purified cell-wall peroxidase from French bean (Phaseolus vulgaris L.). Biochem. J. 299: 747–753.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Patykowski, J. Role of hydrogen peroxide and apoplastic peroxidase in tomato — Botrytis cinerea interaction. Acta Physiol Plant 28, 589–598 (2006). https://doi.org/10.1007/s11738-006-0054-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-006-0054-6