Abstract
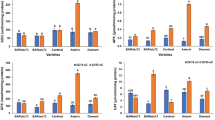
Changes in the activities of peroxidase, ascorbate peroxidase, catalase and superoxide dismutase in rice in response to infection by Rhizoctonia solani were studied. A significant increase in peroxidase activity was observed in R. solani-inoculated rice leaf sheaths 1 day after inoculation and the maximum enzyme activity was recorded 3 days after inoculation at which period a 3-fold increase in peroxidase activity was observed compared to the untreated control. Three peroxidase isozymes viz., PO-4, PO-5 and PO-6 were induced in rice upon infection by R. solani. Ascorbate peroxidase and catalase activities significantly increased 1–2 days after inoculation and the maximum enzyme activities were recorded 5 days after inoculation. Superoxide dismutase activity increased significantly 2 days after inoculation and increased progressively, reaching four times the control value at 7 days after inoculation.
Similar content being viewed by others
References
Asada K. 1984. Chloroplast: Formation of active oxygen and its scavenging. Meth. Enzymol. 105: 422–429.
Baker C. J. and Orlandi E. W. 1995. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33: 299–321.
Belid El-Moshaty F. I. B., Pike S. M., Novacky A. J. and Sehgal O. P. 1993. Lipid peroxidation and superoxide production in cowpea (Vigna unguiculata) leaves infected with tobacco ring spot virus or southern bean mosaic virus. Physiol. Mol. Plant Pathol. 43: 109–119.
Bradley D. J., Kjellbom P. and Lamb C. J. 1992. Elicitor-and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70: 21–30.
Buonaurio R., Torre G. D. and Montalbini P. 1987. Soluble superoxide dismutase (SOD) in susceptible and resistant host-parasite complexes of Phaseolus vulgaris and Uromyces phaseoli. Physiol. Mol. Plant Pathol. 31: 173–184.
Chance B. and Maehly A. C. 1955. Assay of catalases and peroxidases. Meth. Enzymol. 2: 764–775.
Conrath U., Chen Z., Ricigliano J. W. and Klessig D. F. 1995. Two inducers of plant defense responses, 2-6-dichloroisonicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc. Natl. Acad. Sci. USA. 92: 7143–7147.
Dixon R. A. and Harrison M. J. 1990. Activation, structure and organisation of genes involved in microbial defense in plants. Adv. Gent. 28: 165–234.
Durner J. and Klessig D. F. 1995. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl. Acad. Sci. USA. 92: 11312–11316.
El-Zahaby H. M., Gullner G. and Kiraly Z. 1995. Effects of powdery mildew infection of barley on the ascorbate-glutathione cycle and other antioxidants in different host-pathogen interactions. Phytopathology 85: 1225–1230.
Flott B. E., Moerschbacher B. M. and Reisener H. 1989. Peroxidase isoenzyme patterns of resistant and susceptible wheat leaves following stem rust infection. New Phytol. 111: 413–421.
Fodor J., Gullner G., Adam A. L., Barna B., Komives T. and Kiraly Z. 1997. Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco. Role in systemic acquired resistance. Plant Physiol. 114: 1443–1451.
Foyer C. H., Descourvieress P. and Kunert K. J. 1994. Protection against oxygen radicals: An important defense mechanism studied in transgenic plants. Plant cell Environ. 17: 507–523.
Giannopolitis C. N. and Ries S. K. 1977. Superoxide dismutase. Plant Physiol. 59: 309–314.
Gonner M. V. and Schlosser E. 1993. Oxidative stress in interactions between Avena sativa L. and Drechslera spp. Physiol. Mol. Plant Pathol. 42: 221–234.
Grant J. J. and Loake G. J. 2000. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 124: 21–29.
Lamb C. and Dixon R. A. 1997. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 251–275.
Lu H. and Higgins V. J. 1998. Measurement of active oxygen species generated in planta in response to elicitor AVR9 of Cladosporium fulvum. Physiol. Mol. Plant Pathol. 52: 35–51.
Mehdy M.C. 1994. Active oxygen species in plant defense against pathogens. Plant Physiol. 105: 467–472.
Milosevic N. and Slusarenko A. J. 1996. Active oxygen metabolism and lignification in the hypersensitive response in bean. Physiol. Mol. Plant Pathol. 49: 143–158.
Nadolny L. and Sequeira L. 1980. Increase in peroxidase activities are not directly involved in induced resistance in tobacco. Physiol. Plant Pathol. 16: 1–8.
Riedle-Bauer M. 2000. Role of reactive oxygen species and antioxidant enzymes in systemic virus infections of plants. J. Phytopathol. 148: 297–302.
Scandalios J. G. 1993. Oxygen stress and superoxide dismutases. Plant Physiol. 101: 7–12.
Sridhar R. and Ou S. H. 1974. Biochemical changes associated with the development of resistant and susceptible types of rice blast lesions. Phytopathol. Z. 79: 222–230.
Srivastava S. K. 1987. Peroxidase and polyphenol oxidase in Brassica juncea infected with Macrophomina phaseolina (Tassi) Goid. and their implications in disease resistance. J. Phytopathol. 120: 249–254.
Sutherland M. W. 1991. The generation of oxygen free radical during host plant response to infection. Physiol. Mol. Plant Pathol. 39: 79–93
Tzeng D. D. and De Vay J. E. 1993. Role of oxygen radicals in plant disease development. Adv. Plant Pathol. 10: 1–34.
Vera-Estrella R., Blumwald E. and Higgins V. J. 1993. Non-specific glycopeptide elicitors of Cladosporium fulvum: evidence for involvement of active oxygen species in elicitor-induced effects on tomato cell suspensions. Physiol. Mol. Plant Pathol. 42: 9–22.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paranidharan, V., Palaniswami, A., Vidhyasekaran, P. et al. Induction of enzymatic scavengers of active oxygen species in rice in response to infection by Rhizoctonia solani . Acta Physiol Plant 25, 91–96 (2003). https://doi.org/10.1007/s11738-003-0041-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-003-0041-0