Abstract
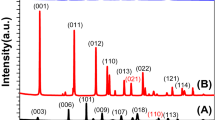
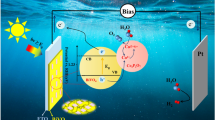
Hydrogen production from photoelectrochemical (PEC) water splitting has been regarded as a promising way to utilize renewable and endless solar energy. However, semiconductor film grown on photoelectrode suffers from numerous challenges, leading to the poor PEC performance. Herein, a straightforward sol-gel method with the ligand-induced growth strategy was employed to obtain dense and homogeneous copper bismuthate photocathodes for PEC hydrogen evolution reaction. By various characterizations, it was found that the nucleation and surface growth of CuBi2O4 layer induced by 2-methoxyethanol ligand (2-CuBi2O4) demonstrated a decent crystallinity and coverage, as well as a large grain size and a low oxygen vacancy concentration, leading to the good ability of light absorption and carrier migration. Consequently, under simulated sunlight irradiation (AM1.5G, 100 mW/cm2), the 2-CuBi2O4 photocathode achieved an enhanced photocurrent density of −1.34 mA·cm−2 at 0.4 V versus the reversible hydrogen electrode and a promising applied bias photon-to-current efficiency of 0.586%. This surface modification by ligand growth strategy will shed light on the future design of advanced photoelectrodes for PEC water splitting.

Similar content being viewed by others
References
Zhou P, Navid I A, Ma Y, et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature, 2023, 613(7942): 66–70
Huang C, Zhou Q, Duan D, et al. The rapid self-reconstruction of Fe-modified Ni hydroxysulfide for efficient and stable large-current-density water/seawater oxidation. Energy & Environmental Science, 2022, 15(11): 4647–4658
Song H, Luo S, Huang H, et al. Solar-driven hydrogen production: Recent advances, challenges, and future perspectives. ACS Energy Letters, 2022, 7(3): 1043–1065
Zhou B, Sun S. Approaching the commercial threshold of solar water splitting toward hydrogen by III-nitrides nanowires. Frontiers in Energy, 2023, online, https://doi.org/10.1007/s11708-023-0870-z
Guerra O J, Eichman J, Kurtz J, et al. Cost competitiveness of electrolytic hydrogen. Joule, 2019, 3(10): 2425–2443
Terlouw T, Bauer C, McKenna R, et al. Large-scale hydrogen production via water electrolysis: A techno-economic and environmental assessment. Energy & Environmental Science, 2022, 15(9): 3583–3602
Hosseini S E, Wahid M A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renewable & Sustainable Energy Reviews, 2016, 57: 850–866
Zhou Q, Liao L, Zhou H, et al. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis. Materials Today Physics, 2022, 26: 100727
Rashid R T, Chen Y, Liu X, et al. Tunable green syngas generation from CO2 and H2O with sunlight as the only energy input. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(26): e2121174119
Yu L, Wu L, McElhenny B, et al. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy & Environmental Science, 2020, 13(10): 3439–3446
Zhou H, Zhang D, Gong X, et al. A dual-ligand strategy to regulate the nucleation and growth of lead chromate photoanodes for photoelectrochemical water splitting. Advanced Materials, 2022, 34(29): 2110610
Wang Z, Sheng B, Chen Y, et al. Photocatalytic syngas production from bio-derived glycerol and water on AuIn-decorated GaN nanowires supported by Si wafer. Green Chemistry, 2023, 25(1): 288–295
Li H, Liu H, Wang F, et al. Hot electron assisted photoelectrochemical water splitting from Au-decorated ZnO@TiO2 nanorods array. Nano Research, 2022, 15(7): 5824–5830
Masoumi Z, Tayebi M, Kolaei M, et al. Efficient and stable coreshell α–Fe2O3/WS2/WOx photoanode for oxygen evolution reaction to enhance photoelectrochemical water splitting. Applied Catalysis B: Environmental, 2022, 313: 121447
Bagal I V, Jun S, Choi M, et al. Investigation of charge carrier dynamics in beaded ZnO nanowire decorated with SnS2/IrOx cocatalysts for enhanced photoelectrochemical water splitting. Applied Surface Science, 2023, 613: 156091
Kong H, Yang H, Park J S, et al. Spatial control of oxygen vacancy concentration in monoclinic WO3 photoanodes for enhanced solar water splitting. Advanced Functional Materials, 2022, 32(36): 2204106
Li Y, Dai X, Bu Y, et al. Photoelectrochemical performance improving mechanism: Hybridization appearing at the energy band of BiVO4 photoanode by doped quantum layers modification. Small, 2022, 18(21): 2200454
Mary A S, Murugan C, Pandikumar A. Uplifting the charge carrier separation and migration in Co-doped CuBi2O4/TiO2 p-n heterojunction photocathode for enhanced photoelectrocatalytic water splitting. Journal of Colloid and Interface Science, 2022, 608: 2482–2492
Shin D, Saparov B, Mitzi D B. Defect engineering in multinary earth-abundant chalcogenide photovoltaic materials. Advanced Energy Materials, 2017, 7(11): 1602366
Malyi O I, Zunger A. False metals, real insulators, and degenerate gapped metals. Applied Physics Reviews, 2020, 7(4): 041310
Li C, He J, Xiao Y, et al. Earth-abundant Cu-based metal oxide photocathodes for photoelectrochemical water splitting. Energy & Environmental Science, 2020, 13(10): 3269–3306
Alizadeh A, Roudgar-Amoli M, Bonyad-Shekalgourabi S M. Dye sensitized solar cells go beyond using perovskite and spinel inorganic materials: A review. Renewable & Sustainable Energy Reviews, 2022, 157: 112047
Wu T, Du Y, Dai L, et al. A direct Z-scheme AgBr/CuBi2O4 photocathode for ultrasensitive detection of ciprofloxacin and ofloxacin by controlling the release of luminol in self-powered microfluidic photoelectrochemical aptasensors. Analytical Chemistry, 2022, 94(30): 10651–10658
Reddy D A, Kim Y, Varma P, et al. Inverse opal CuBi2O4 photocathodes for robust photoelectrochemical water splitting. ACS Applied Energy Materials, 2022, 5(5): 6050–6058
Cao H, Zhang Z, Zhang M, et al. The effect of defects in tin-based perovskites and their photovoltaic devices. Materials Today Physics, 2021, 21: 100513
Kang D, Hill J C, Park Y, et al. Photoelectrochemical properties and photostabilities of high surface area CuBi2O4 and Ag-doped CuBi2O4 photocathodes. Chemistry of Materials, 2016, 28(12): 4331–4340
Oh W D, Lua S K, Dong Z, et al. Rational design of hierarchically-structured CuBi2O4 composites by deliberate manipulation of the nucleation and growth kinetics of CuBi2O4 for environmental applications. Nanoscale, 2016, 8(4): 2046–2054
Shen X, Zhu Z, Zhang H, et al. Novel sphere-like copper bismuth oxide fabricated via ethylene glycol-introduced solvothermal method with improved adsorptive and photocatalytic performance in sulfamethazine removal. Environmental Science and Pollution Research International, 2022, 29(31): 47159–47173
Xu Y, Jian J, Li F, et al. Porous CuBi2O4 photocathodes with rationally engineered morphology and composition towards high-efficiency photoelectrochemical performance. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 2019, 7(38): 21997–22004
Wang Y, Hu J, Liu S, et al. Influence of grain size on photoelectrocatalytic performance of CuBi2O4 photocathodes. International Journal of Hydrogen Energy, 2022, 47(89): 37774–37782
Yang Y, He A, Yang M, et al. Selective electroreduction of CO2 to ethanol over a highly stable catalyst derived from polyaniline/CuBi2O4. Catalysis Science & Technology, 2021, 11(17): 5908–5916
Kim J H, Adishev A, Kim J, et al. All-bismuth-based oxide tandem cell for solar overall water splitting. ACS Applied Energy Materials, 2018, 1(12): 6694–6699
Jaiswal M K, Carrow J K, Gentry J L, et al. Vacancy-driven gelation using defect-rich nanoassemblies of 2D transition metal dichalcogenides and polymeric binder for biomedical applications. Advanced Materials, 2017, 29(36): 1702037
Mohamed H S H, Rabia M, Zhou X G, et al. Phase-junction Ag/TiO2 nanocomposite as photocathode for H2 generation. Journal of Materials Science and Technology, 2021, 83: 179–187
Seo G, Kim B, Hwang S W, et al. High-performance bulky crystalline copper bismuthate photocathode for enhanced solar water splitting. Nano Energy, 2021, 80: 105568
Li M, Tian X, Zou X, et al. Promoting photoelectrochemical hydrogen evolution activity of CuBi2O4 photocathode through ramping rate control. International Journal of Hydrogen Energy, 2020, 45(30): 15121–15128
Guo F, Shi W, Wang H, et al. Facile fabrication of a CoO/g-C3N4 p–n heterojunction with enhanced photocatalytic activity and stability for tetracycline degradation under visible light. Catalysis Science & Technology, 2017, 7(15): 3325–3331
Li X, Yu J, Low J, et al. Engineering heterogeneous semiconductors for solar water splitting. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 2015, 3(6): 2485–2534
Wan Z, Mao Q, Chen Q. Proton-dependent photocatalytic dehalogenation activities caused by oxygen vacancies of In2O3. Chemical Engineering Journal, 2021, 403: 126389
Lindberg A E, Wang W, Zhang S, et al. Can a PbCrO4 photoanode perform as well as isoelectronic BiVO4? ACS Applied Energy Materials, 2020, 3(9): 8658–8666
Freysoldt C, Grabowski B, Hickel T, et al. First-principles calculations for point defects in solids. Reviews of Modern Physics, 2014, 86(1): 253–305
Kedar L, Bond C E, Muirhead D. Carbon ordering in an aseismic shear zone: Implications for Raman geothermometry and strain tracking. Earth and Planetary Science Letters, 2020, 549: 116536
Varanasi C, Petry J, Brunke L, et al. Growth of high-quality carbon nanotubes on free-standing diamond substrates. Carbon, 2010, 48(9): 2442–2446
Pang Y X, Li X, Zhang X, et al. The synthesis of carbon-based quantum dots: A supercritical fluid approach and perspective. Materials Today Physics, 2022, 27: 100752
Bourque A J, Rutledge G C. Heterogeneous nucleation of an n-alkane on graphene-like materials. European Polymer Journal, 2018, 104: 64–71
Khaleghi A, Sadrameli S M, Manteghian M. Thermodynamic and kinetics investigation of homogeneous and heterogeneous nucleation. Reviews in Inorganic Chemistry, 2020, 40(4): 167–192
Wang F, Chemseddine A, Abdi F F, et al. Spray pyrolysis of CuBi2O4 photocathodes: improved solution chemistry for highly homogeneous thin films. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 2017, 5(25): 12838–12847
Song A, Plate P, Chemseddine A, et al. Cu:NiO as a hole-selective back contact to improve the photoelectrochemical performance of CuBi2O4 thin film photocathodes. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 2019, 7(15): 9183–9194
Kang D, Hill J C, Park Y, et al. Photoelectrochemical properties and photostabilities of high surface area CuBi2O4 and Ag-doped CuBi2O4 photocathodes. Chemistry of Materials, 2016, 28(12): 4331–4340
Hahn N T, Holmberg V C, Korgel B A, et al. Electrochemical synthesis and characterization of p-CuBi2O4 thin film photocathodes. Journal of Physical Chemistry C, 2012, 116(10): 6459–6466
Berglund S P, Abdi F F, Bogdanoff P, et al. Comprehensive evaluation of CuBi2O4 as a photocathode material for photoelectrochemical water splitting. Chemistry of Materials, 2016, 28(12): 4231–4242
Li J, Griep M, Choi Y, et al. Photoelectrochemical overall water splitting with textured CuBi2O4 as a photocathode. Chemical Communications, 2018, 54(27): 3331–3334
Choi Y H, Yang K D, Kim D H, et al. p-Type CuBi2O4 thin films prepared by flux-mediated one-pot solution process with improved structural and photoelectrochemical characteristics. Materials Letters, 2017, 188: 192–196
Acknowledgements
This work was financially supported by the National Key R&D Program of China (Grant No. 2022YFB3803600), the National Natural Science Foundation of China (Grant No. U20A20246), and the Fundamental Research Funds for the Central Universities, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wang, Z., Wu, Q., Wang, J. et al. Surface modification by ligand growth strategy for dense copper bismuth film as photocathode to enhance hydrogen production activity. Front. Energy 18, 89–100 (2024). https://doi.org/10.1007/s11708-023-0893-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11708-023-0893-5