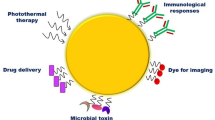
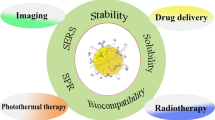
Abstract
Regarding the increasing number of cancer patients, the global burden of this disease is continuing to grow. Despite a considerable improvement in the diagnosis and treatment of various types of cancer, new diagnosis and treatment strategies are required. Nanotechnology, as an interesting and advanced field in medicine, is aimed to further advance both cancer diagnosis and treatment. Gold nanocages (AuNCs), with hollow interiors and porous walls, have received a great deal of interest in various biomedical applications such as diagnosis, imaging, drug delivery, and hyperthermia therapy due to their special physicochemical characteristics including the porous structure and surface functionalization as well as optical and photothermal properties. This review is focused on recent developments in therapeutic and diagnostic and applications of AuNCs with an emphasis on their theranostic applications in cancer diseases.
Similar content being viewed by others
References
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer, 2019, 144(8): 1941–1953
Miller K D, Siegel R L, Lin C C, et al. Cancer treatment and survivorship statistics, 2016. CA: a Cancer Journal for Clinicians, 2016, 66(4): 271–289
Abedi M, Abolmaali S S, Abedanzadeh M, et al. Core-shell imidazoline-functionalized mesoporous silica superparamagnetic hybrid nanoparticles as a potential theranostic agent for controlled delivery of platinum(II) compound. International Journal of Nanomedicine, 2020, 15: 2617–2631
Abedi M, Abolmaali S S, Abedanzadeh M, et al. Citric acid functionalized silane coupling versus post-grafting strategy for dual pH and saline responsive delivery of cisplatin by Fe3O4/carboxyl functionalized mesoporous SiO2 hybrid nanoparticles: A-synthesis, physicochemical and biological characterization. Materials Science and Engineering C, 2019, 104: 109922
Abedi M, Abolmaali S S, Heidari R, et al. Hierarchical mesoporous zinc-imidazole dicarboxylic acid MOFs: Surfactant-directed synthesis, pH-responsive degradation, and drug delivery. International Journal of Pharmaceutics, 2021, 602: 120685
Chaturvedi V K, Singh A, Singh V K, et al. Cancer nanotechnology: A new revolution for cancer diagnosis and therapy. Current Drug Metabolism, 2019, 20(6): 416–429
Alimardani V, Abolmaali S S, Tamaddon A M, et al. Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Delivery and Translational Research, 2021, 11(3): 788–816
Fruscella M, Ponzetto A, Crema A, et al. The extraordinary progress in very early cancer diagnosis and personalized therapy: The role of oncomarkers and nanotechnology. Journal of Nanotechnology, 2016, 2016: 1–18
Kaushik A, Jayant R D, Nair M, eds. Advances in Personalized Nanotherapeutics. Springer, 2017
Grobmyer S R, Iwakuma N, Sharma P, et al. What is cancer nanotechnology? In: Cancer Nanotechnology: Methods and Protocols. Methods in Molecular Biology, 2010, 624: 1–9
Jafari M, Abolmaali S S, Najafi H, et al. Hyperbranched polyglycerol nanostructures for anti-biofouling, multifunctional drug delivery, bioimaging and theranostic applications. International Journal of Pharmaceutics, 2020, 576: 118959
Akhter S, Ahmad M Z, Ahmad F J, et al. Gold nanoparticles in theranostic oncology: Current state-of-the-art. Expert Opinion on Drug Delivery, 2012, 9(10): 1225–1243
Chan W C W, Khademhosseini A, Parak W, et al. Cancer: Nanoscience and nanotechnology approaches. ACS Nano, 2017, 11(5): 4375–4376
Alimardani V, Abolmaali S S, Yousefi G, et al. Microneedle arrays combined with nanomedicine approaches for transdermal delivery of therapeutics. Journal of Clinical Medicine, 2021, 10 (2): 181–214
Brown S C, Palazuelos M, Sharma P, et al. Nanoparticle characterization for cancer nanotechnology and other biological applications. In: Cancer Nanotechnology: Methods and Protocols. Methods in Molecular Biology, 2010, 624: 39–65
Lin G, Chen S, Mi P. Nanoparticles targeting and remodeling tumor microenvironment for cancer theranostics. Journal of Biomedical Nanotechnology, 2018, 14(7): 1189–1207
Rawal S, Patel M M J. Threatening cancer with nanoparticle aided combination oncotherapy. Journal of Controlled Release, 2019, 301(301): 76–109
Borandeh S, Alimardani V, Abolmaali S S, et al. Graphene family nanomaterials in Ocular applications: Physicochemical properties and toxicity. Chemical Research in Toxicology, 2021, 34(6): 1386–1402
Taghizadeh S, Alimardani V, Roudbali P L, et al. Gold nanoparticles application in liver cancer. Photodiagnosis and Photodynamic Therapy, 2019, 25: 389–400
Connor E E, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small, 2005, 1(3): 325–327
Venditti I. Gold nanoparticles in photonic crystals applications: A review. Materials, 2017, 10(2): 97–115
Elahi N, Kamali M, Baghersad M H J T. Recent biomedical applications of gold nanoparticles: A review. Talanta, 2018, 184: 537–556
Huang X, El-Sayed M A J. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. Journal of Advanced Research, 2010, 1(1): 13–28
Kang M S, Lee S Y, Kim K S, et al. State of the art biocompatible gold nanoparticles for cancer theragnosis. Pharmaceutics, 2020, 12(8): 701–723
Bai X, Wang Y, Song Z, et al. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. International Journal of Molecular Sciences, 2020, 21 (7): 2480–2497
Skrabalak S E, Au L, Lu X, et al. Gold nanocages for cancer detection and treatment. Nanomedicine, 2007, 2(5): 657–668
Xia X, Xia Y. Gold nanocages as multifunctional materials for nanomedicine. Frontiers in Physics, 2014, 9(3): 378–384
Yao H, Long X, Cao L, et al. Multifunctional ferritin nanocages for bimodal imaging and targeted delivery of doxorubicin into cancer cells. RSC Advances, 2016, 6(111): 109322–109333
Jiang B, Yan L, Zhang J, et al. Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. ACS Applied Materials & Interfaces, 2019, 11(10): 9747–9755
Kosaki Y, Izawa H, Ishihara S, et al. Nanoporous carbon sensor with cage-in-fiber structure: Highly selective aniline adsorbent toward cancer risk management. ACS Applied Materials & Interfaces, 2013, 5(8): 2930–2934
Luo Y, Wang X, Du D, et al. Hyaluronic acid-conjugated apoferritin nanocages for lung cancer targeted drug delivery. Biomaterials Science, 2015, 3(10): 1386–1394
Hood Z D, Kubelick K P, Gilroy K D, et al. Photothermal transformation of Au-Ag nanocages under pulsed laser irradiation. Nanoscale, 2019, 11(6): 3013–3020
Wang Y, Xiao Y, Zhou H, et al. Ultra-high payload of doxorubicin and pH-responsive drug release in CuS nanocages for a combination of chemotherapy and photothermal therapy. RSC Advances, 2013, 3(45): 23133–23138
Wang T, Zhang L, Su Z, et al. Multifunctional hollow mesoporous silica nanocages for cancer cell detection and the combined chemotherapy and photodynamic therapy. ACS Applied Materials & Interfaces, 2011, 3(7): 2479–2486
Huang S, Li C, Wang W, et al. A54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. International Journal of Nanomedicine, 2017, 12: 5163–5176
Raniolo S, Vindigni G, Ottaviani A, et al. Selective targeting and degradation of doxorubicin-loaded folate-functionalized DNA nanocages. Nanomedicine: Nanotechnology, Biology and Medicine, 2018, 14(4): 1181–1190
Skrabalak S E, Chen J, Sun Y, et al. Gold nanocages: Synthesis, properties, and applications. Accounts of Chemical Research, 2008, 41(12): 1587–1595
Hu F, Zhang Y, Chen G, et al. Double-walled Au nanocage/SiO2 nanorattles: Integrating SERS imaging, drug delivery and photothermal therapy. Small, 2015, 11(8): 985–993
Cabuzu D, Cirja A, Puiu R, et al. Biomedical applications of gold nanoparticles. Current Topics in Medicinal Chemistry, 2015, 15 (16): 1605–1613
Behnam M A, Emami F, Sobhani Z, et al. Novel combination of silver nanoparticles and carbon nanotubes for plasmonic photo thermal therapy in melanoma cancer model. Advanced Pharmaceutical Bulletin, 2018, 8(1): 49–55
Van de Broek B, Frederix F, Bonroy K, et al. Shape-controlled synthesis of NIR absorbing branched gold nanoparticles and morphology stabilization with alkanethiols. Nanotechnology, 2011, 22(1): 015601
Yang X, Skrabalak S E, Li Z Y, et al. Photoacoustic tomography of a rat cerebral cortex in vivo with Au nanocages as an optical contrast agent. Nano Letters, 2007, 7(12): 3798–3802
Kim C, Cho E C, Chen J, et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano, 2010, 4(8): 4559–4564
Cho E C, Zhang Y, Cai X, et al. Quantitative analysis of the fate of gold nanocages in vitro and in vivo after uptake by U87-MG tumor cells. Angewandte Chemie International Edition, 2013, 52 (4): 1152–1155
Cai X, Li W, Kim C H, et al. In vivo quantitative evaluation of the transport kinetics of gold nanocages in a lymphatic system by noninvasive photoacoustic tomography. ACS Nano, 2011, 5(12): 9658–9667
Song K H, Kim C, Cobley C M, et al. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Letters, 2009, 9(1): 183–188
Cang H, Sun T, Li Z Y, et al. Gold nanocages as contrast agents for spectroscopic optical coherence tomography. Optics Letters, 2005, 30(22): 3048–3050
Wen S, Miao X, Fan G C, et al. Aptamer-conjugated Au nanocage/SiO2 core-shell bifunctional nanoprobes with high stability and biocompatibility for cellular SERS imaging and near-infrared photothermal therapy. ACS Sensors, 2019, 4(2): 301–308
Cao X, Wang Z, Bi L, et al. Gold nanocage-based surface-enhanced Raman scattering probes for long-term monitoring of intracellular microRNA during bone marrow stem cell differentiation. Nanoscale, 2020, 12(3): 1513–1527
Yang Y, Fu Y, Su H, et al. Sensitive detection of MCF-7 human breast cancer cells by using a novel DNA-labeled sandwich electrochemical biosensor. Biosensors & Bioelectronics, 2018, 122: 175–182
Chen M, Wu D, Tu S, et al. A novel biosensor for the ultrasensitive detection of the lncRNA biomarker MALAT1 in non-small cell lung cancer. Scientific Reports, 2021, 11(1): 3666
Wang Y, Xu J, Xia X, et al. SV119-gold nanocage conjugates: A new platform for targeting cancer cells via sigma-2 receptors. Nanoscale, 2012, 4(2): 421–424
Mackey M A, Saira F, Mahmoud M A, et al. Inducing cancer cell death by targeting its nucleus: Solid gold nanospheres versus hollow gold nanocages. Bioconjugate Chemistry, 2013, 24(6): 897–906
Chen J, Wang D, Xi J, et al. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Letters, 2007, 7(5): 1318–1322
Liang R, Xie J, Li J, et al. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials, 2017, 149: 41–50
Zhu D M, Xie W, Xiao Y S, et al. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnology, 2018, 29(8): 084002–084017
Yang J, Shen D, Zhou L, et al. Spatially confined fabrication of core-shell gold nanocages@mesoporous silica for near-infrared controlled photothermal drug release. Chemistry of Materials, 2013, 25(15): 3030–3037
He H, Liu L, Zhang S, et al. Smart gold nanocages for mild heat-triggered drug release and breaking chemoresistance. Journal of Controlled Release, 2020, 323: 387–397
Zhang Z, Wang Y, Xu S, et al. Photothermal gold nanocages filled with temperature sensitive tetradecanol and encapsulated with glutathione responsive polycurcumin for controlled DOX delivery to maximize anti-MDR tumor effects. Journal of Materials Chemistry B: Materials for Biology and Medicine, 2017, 5(27): 5464–5472
Shi P, Qu K, Wang J, et al. pH-responsive NIR enhanced drug release from gold nanocages possesses high potency against cancer cells. Chemical Communications, 2012, 48(61): 7640–7642
Gao L, Fei J, Zhao J, et al. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano, 2012, 6(9): 8030–8040
Hu Y, Huang S, Zhao X, et al. Preparation of photothermal responsive and ROS generative gold nanocages for cancer therapy. Chemical Engineering Journal, 2021, 421: 129744
Wang S, Song Y, Cao K, et al. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomaterialia, 2021 (in press)
Pakravan A, Azizi M, Rahimi F, et al. Comparative effect of thermo/pH-responsive polymer-coated gold nanocages and hollow nanostars on chemo-photothermal therapy of breast cancer cells. Cancer Nanotechnology, 2021, 12(1): 19
Sun M, Duan Y, Ma Y, et al. Cancer cell-erythrocyte hybrid membrane coated gold nanocages for near infrared light-activated photothermal/radio/chemotherapy of breast cancer. International Journal of Nanomedicine, 2020, 15: 6749–6760
Battogtokh G, Gotov O, Kang J H, et al. Glycol chitosan-coated near-infrared photosensitizer-encapsulated gold nanocages for glioblastoma phototherapy. Nanomedicine: Nanotechnology, Biology, and Medicine, 2019, 18: 315–325
Feng Y, Cheng Y, Chang Y, et al. Time-staggered delivery of erlotinib and doxorubicin by gold nanocages with two smart polymers for reprogrammable release and synergistic with photothermal therapy. Biomaterials, 2019, 217: 119327
Yu Y, Zhang Z, Wang Y, et al. A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy. Acta Biomaterialia, 2017, 59: 170–180
Srivatsan A, Jenkins S V, Jeon M, et al. Gold nanocage-photosensitizer conjugates for dual-modal image-guided enhanced photodynamic therapy. Theranostics, 2014, 4(2): 163–174
Farahavar G, Abolmaali S S, Nejatollahi F, et al. Single-chain antibody-decorated Au nanocages@liposomal layer nanoprobes for targeted SERS imaging and remote-controlled photothermal therapy of melanoma cancer cells. Materials Science and Engineering C, 2021, 124: 112086
Xu X, Chong Y, Liu X, et al. Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta Biomaterialia, 2019, 84: 328–338
Fang X, Lui K H, Li S, et al. Multifunctional nanotheranostic gold nanocage/selenium core-shell for PAI-guided chemophotothermal synergistic therapy in vivo. International Journal of Nanomedicine, 2020, 15: 10271–1
Jiang H. Photoacoustic Tomography. CRC Press, 2018
Upputuri P K, Pramanik M. Recent advances toward preclinical and clinical translation of photoacoustic tomography: A review. Journal of Biomedical Optics, 2016, 22(4): 041006
Wang L V, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nature Methods, 2016, 13(8): 627–638
Li W, Brown P K, Wang L V, et al. Gold nanocages as contrast agents for photoacoustic imaging. Contrast Media & Molecular Imaging, 2011, 6(5): 370–377
Bhandari A, Xia E, Wang Y, et al. Impact of sentinel lymph node biopsy in newly diagnosed invasive breast cancer patients with suspicious node: A comparative accuracy survey of fine-needle aspiration biopsy versus core-needle biopsy. American Journal of Translational Research, 2018, 10(6): 1860–1873
Spaide R F, Fujimoto J G, Waheed N K, et al. Optical coherence tomography angiography. Progress in Retinal and Eye Research, 2018, 64: 1–55
Tan A C S, Tan G S, Denniston A K, et al. An overview of the clinical applications of optical coherence tomography angiography. Eye, 2018, 32(2): 262–286
Li M, Landahl S, East A R, et al. Optical coherence tomography — A review of the opportunities and challenges for postharvest quality evaluation. Postharvest Biology and Technology, 2019, 150: 9–18
Au L, Zhang Q, Cobley C M, et al. Quantifying the cellular uptake of antibody-conjugated Au nanocages by two-photon microscopy and inductively coupled plasma mass spectrometry. ACS Nano, 2010, 4(1): 35–42
Jin C, Liang F, Wang J, et al. Rational design of cyclometalated iridium(III) complexes for three-photon phosphorescence bio-imaging. Angewandte Chemie International Edition, 2020, 59 (37): 15987–15991
Pang B, Yang X, Xia Y. Putting gold nanocages to work for optical imaging, controlled release and cancer theranostics. Nanomedicine, 2016, 11(13): 1715–1728
Chen Y, Zhang Y, Liang W, et al. Gold nanocages as contrast agents for two-photon luminescence endomicroscopy imaging. Nanomedicine: Nanotechnology, Biology, and Medicine, 2012, 8 (8): 1267–1270
Govindaraju S, Yun K. Synthesis of gold nanomaterials and their cancer-related biomedical applications: An update. 3Biotech, 2018, 8: 113
Wu Y, Leng Y, Xi J, et al. Scanning all-fiber-optic endomicroscopy system for 3D nonlinear optical imaging of biological tissues. Optics Express, 2009, 17(10): 7907–7915
Tong L, Cobley C M, Chen J, et al. Bright three-photon luminescence from gold/silver alloyed nanostructures for bioimaging with negligible photothermal toxicity. Angewandte Chemie International Edition, 2010, 49(20): 3485–3488
Auner G W, Koya S K, Huang C, et al. Applications of Raman spectroscopy in cancer diagnosis. Cancer and Metastasis Reviews, 2018, 37(4): 691–717
Zong C, Xu M, Xu L J, et al. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chemical Reviews, 2018, 118(10): 4946–4980
Bruzas I, Lum W, Gorunmez Z, et al. Advances in surface-enhanced Raman spectroscopy (SERS) substrates for lipid and protein characterization: Sensing and beyond. The Analyst, 2018, 143(17): 3990–4008
Ding S Y, You E M, Tian Z Q, et al. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chemical Society Reviews, 2017, 46(13): 4042–4076
Indrasekara A S, Paladini B J, Naczynski D J, et al. Dimeric gold nanoparticle assemblies as tags for SERS-based cancer detection. Advanced Healthcare Materials, 2013, 2(10): 1370–1376
Jorgenson E, Ianoul A. Biofunctionalization of plasmonic nanoparticles with short peptides monitored by SERS. The Journal of Physical Chemistry B, 2017, 121(5): 967–974
Cao X, Shan Y, Tan L, et al. Hollow Au nanoflower substrates for identification and discrimination of the differentiation of bone marrow mesenchymal stem cells by surface-enhanced Raman spectroscopy. Journal of Materials Chemistry B: Materials for Biology and Medicine, 2017, 5(30): 5983–5995
Sun D, Xu W, Liang C, et al. Smart surface-enhanced resonance Raman scattering nanoprobe for monitoring cellular alkaline phosphatase activity during osteogenic differentiation. ACS Sensors, 2020, 5(6): 1758–1767
Huang X, Jain P K, El-Sayed I H, et al. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers in Medical Science, 2008, 23(3): 217–228
Chen Y, Li L, Chen W, et al. Near-infrared small molecular fluorescent dyes for photothermal therapy. Chinese Chemical Letters, 2019, 30(7): 1353–1360
Song X, Chen Q, Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Research, 2015, 8(2): 340–354
Wang Y, Black K C, Luehmann H, et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano, 2013, 7(3): 2068–2077
Vines J B, Yoon J H, Ryu N E, et al. Gold nanoparticles for photothermal cancer therapy. Frontiers in Chemistry, 2019, 7: 167–183
Chen J, Glaus C, Laforest R, et al. Gold nanocages as photothermal transducers for cancer treatment. Small, 2010, 6 (7): 811–817
Peer D, Karp J M, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology, 2007, 2 (12): 751–760
Sun Y, Peng Y, Chen Y, et al. Application of artificial neural networks in the design of controlled release drug delivery systems. Advanced Drug Delivery Reviews, 2003, 55(9): 1201–1215
Cui J, Yan Y, Wang Y, et al. Templated assembly of pH-labile polymer-drug particles for intracellular drug delivery. Advanced Functional Materials, 2012, 22(22): 4718–4723
Wang Y, Yan Y, Cui J, et al. Encapsulation of water-insoluble drugs in polymer capsules prepared using mesoporous silica templates for intracellular drug delivery. Advanced Materials, 2010, 22(38): 4293–4297
Yavuz M S, Cheng Y, Chen J, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nature Materials, 2009, 8(12): 935–939
Li H, Li H, Yu W, et al. PEGylated hyaluronidase/NIR induced drug controlled release system for synergetic chemo-photothermal therapy of hepatocellular carcinoma. European Journal of Pharmaceutical Sciences, 2019, 133: 127–136
Her S, Jaffray D A, Allen C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Advanced Drug Delivery Reviews, 2017, 109: 84–101
Hosnedlova B, Kepinska M, Fernandez C, et al. Carbon nanomaterials for targeted cancer therapy drugs: A critical review. Chemical Record, 2019, 19(2–3): 502–522
Pillai G. Nanotechnology toward treating cancer: A comprehensive review. In: Mohapatra S S, Ranjan S, Dasgupta N, et al., eds. Applications of Targeted Nano Drugs and Delivery Systems: Nanoscience and Nanotechnology in Drug Delivery. Micro & Nano Technologies, 2019, 221–256
Pandya T, Patel K K, Pathak R, et al. Liposomal formulations in cancer therapy: Passive versus active targeting. Asian Journal of Pharmaceutical Research and Development, 2019, 7(2): 35–38
Behera A, Padhi S. Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: A review. Environmental Chemistry Letters, 2020, 18(5): 1557–1567
Siminzar P, Omidi Y, Golchin A, et al. Targeted delivery of doxorubicin by magnetic mesoporous silica nanoparticles armed with mucin-1 aptamer. Journal of Drug Targeting, 2020, 28(1): 92–101
Kon I I, Zyubin A Y, Seteikin A Y, et al. FTDT numerical calculatons of local plasmonic fields for multilayer gold nanoparticles-agents for theranostics. In: Andrews D L, Bain A J, Kauranen M, et al., eds. Nanophotonics VIII. Proceedings of SPIE, 2021, 11345: 113452L
Parida S, Maiti C, Rajesh Y, et al. Gold nanorod embedded reduction responsive block copolymer micelle-triggered drug delivery combined with photothermal ablation for targeted cancer therapy. Biochimica et Biophysica Acta: General Subjects, 2017, 1861(1 Pt A): 3039–3052
Yang M, Liu Y, Hou W, et al. Mitomycin C-treated human-induced pluripotent stem cells as a safe delivery system of gold nanorods for targeted photothermal therapy of gastric cancer. Nanoscale, 2017, 9(1): 334–340
Nima Z A, Alwbari A M, Dantuluri V, et al. Targeting nano drug delivery to cancer cells using tunable, multi-layer, silver-decorated gold nanorods. Journal of Applied Toxicology, 2017, 37(12): 1370–1378
Huang S, Duan S, Wang J, et al. Folic-acid-mediated functionalized gold nanocages for targeted delivery of anti-miR-181b in combination of gene therapy and photothermal therapy against hepatocellular carcinoma. Advanced Functional Materials, 2016, 26(15): 2532–2544
Kumar S S D, Mahesh A, Antoniraj M G, et al. Cellular imaging and folate receptor targeting delivery of gum kondagogu capped gold nanoparticles in cancer cells. International Journal of Biological Macromolecules, 2018, 109: 220–230
Burks S R, Ziadloo A, Hancock H A, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One, 2011, 6(9): e24730
Eck W, Craig G, Sigdel A, et al. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS Nano, 2008, 2(11): 2263–2272
Samia A C, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. Journal of the American Chemical Society, 2003, 125(51): 15736–15737
Wu G, Mikhailovsky A, Khant H A, et al. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. Journal of the American Chemical Society, 2008, 130(26): 8175–8177
Acknowledgement
This work was financially supported by the Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure of potential conflict of interest
The authors declare no conflict of interest in this work.
Rights and permissions
About this article
Cite this article
Alimardani, V., Farahavar, G., Salehi, S. et al. Gold nanocages in cancer diagnosis, therapy, and theranostics: A brief review. Front. Mater. Sci. 15, 494–511 (2021). https://doi.org/10.1007/s11706-021-0569-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-021-0569-1