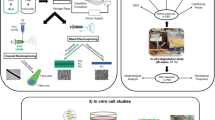
Abstract
Mineralized collagen (MC) is a biomimetic material that mimics natural bone matrix in terms of both chemical composition and microstructure. The biomimetic MC possesses good biocompatibility and osteogenic activity, and is capable of guiding bone regeneration as being used for bone defect repair. However, mechanical strength of existing MC artificial bone is too low to provide effective support at human load-bearing sites, so it can only be used for the repair at non-load-bearing sites, such as bone defect filling, bone graft augmentation, and so on. In the present study, a high strength MC artificial bone material was developed by using collagen as the template for the biomimetic mineralization of the calcium phosphate, and then followed by a cold compression molding process with a certain pressure. The appearance and density of the dense MC were similar to those of natural cortical bone, and the phase composition was in conformity with that of animal’s cortical bone demonstrated by XRD. Mechanical properties were tested and results showed that the compressive strength was comparable to human cortical bone, while the compressive modulus was as low as human cancellous bone. Such high strength was able to provide effective mechanical support for bone defect repair at human load-bearing sites, and the low compressive modulus can help avoid stress shielding in the application of bone regeneration. Both in vitro cell experiments and in vivo implantation assay demonstrated good biocompatibility of the material, and in vivo stability evaluation indicated that this high-strength MC artificial bone could provide long-term effective mechanical support at human load-bearing sites.
Similar content being viewed by others
References
Fu Q, Saiz E, Tomsia A P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomaterialia, 2011, 7(10): 3547–3554
Sakellariou V I, Mavrogenis A F, Babis G C, et al. Comparison of four reconstructive methods for diaphyseal defects of the humerus after tumor resection. Journal of Applied Biomechanics, 2012, 28(5): 568–578
Edwards P K, Ackland T R, Ebert J R. Accelerated weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint: early clinical and radiological outcomes. The American Journal of Sports Medicine, 2013, 41(10): 2314–2324
Thalgott J S, Fogarty M E, Giuffre J M, et al. A prospective, randomized, blinded, single-site study to evaluate the clinical and radiographic differences between frozen and freeze-dried allograft when used as part of a circumferential anterior lumbar interbody fusion procedure. Spine, 2009, 34(12): 1251–1256
Roohani-Esfahani S I, Dunstan C R, Li J J, et al. Unique microstructural design of ceramic scaffolds for bone regeneration under load. Acta Biomaterialia, 2013, 9(6): 7014–7024
Abbah S A, Lam C X, Hutmacher D W, et al. Biological performance of a polycaprolactone-based scaffold used as fusion cage device in a large animal model of spinal reconstructive surgery. Biomaterials, 2009, 30(28): 5086–5093
Wieding J, Fritsche A, Heinl P, et al. Biomechanical behavior of bone scaffolds made of additive manufactured tricalciumphosphate and titanium alloy under different loading conditions. Journal of Applied Biomaterials Functional Materials, 2013, 11(3): 159–166
Kujala S, Tuukkanen J, Jämsä T, et al. Comparison of the bone modeling effects caused by curved and straight nickel — titanium intramedullary nails. Journal of Materials Science: Materials in Medicine, 2002, 13(12): 1157–1161
Behrbalk E, Uri O, Parks R M, et al. Fusion and subsidence rate of stand alone anterior lumbar interbody fusion using PEEK cage with recombinant human bone morphogenetic protein-2. European Spine Journal, 2013, 22(12): 2869–2875
van der Elst M, Klein C P, de Blieck-Hogervorst J M, et al. Bone tissue response to biodegradable polymers used for intra medullary fracture fixation: a long-term in vivo study in sheep femora. Biomaterials, 1999, 20(2): 121–128
Samartzis D, Shen F H, Goldberg E J, et al. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine, 2005, 30(15): 1756–1761
Gibson S, McLeod I, Wardlaw D, et al. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: a randomized control trial. Spine, 2002, 27(15): 1599–1603
Sun L, Hu Y, Ning Z, et al. The correlation between immune rejection and osteoinduction of allogeneic bone grafting. Chinese Medical Journal, 1998, 111(9): 818–822
Benli S, Aksoy S, Havitcioğlu H, et al. Evaluation of bone plate with low-stiffness material in terms of stress distribution. Journal of Biomechanics, 2008, 41(15): 3229–3235
Shi X, Jiang J, Sun L, et al. Hydrolysis and biomineralization of porous PLA microspheres and their influence on cell growth. Colloids and Surfaces B: Biointerfaces, 2011, 85(1): 73–80
Cui F Z, Li Y, Ge J. Self-assembly of mineralized collagen composites. Materials Science and Engineering R: Reports, 2007, 57(1–6): 1–27
Du C, Cui F Z, Zhang W, et al. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. Journal of Biomedical Materials Research, 2000, 50(4): 518–527
Zhang W, Liao S S, Cui F Z. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chemistry of Materials, 2003, 15(16): 3221–3226
Liao S S, Cui F Z, Zhang W, et al. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2004, 69B(2): 158–165
Hvistendahl M. China’s push in tissue engineering. Science, 2012, 338(6109): 900–902
Lian K, Lu H, Guo X, et al. The mineralized collagen for the reconstruction of intra-articular calcaneal fractures with trabecular defects. Biomatter, 2013, 3(4): e27250 (5 pages)
Yu X, Xu L, Cui F Z, et al. Clinical evaluation of mineralized collagen as a bone graft substitute for anterior cervical intersomatic fusion. Journal of Biomaterials and Tissue Engineering, 2012, 2(2): 170–176
Lam C X F, Savalani M M, Teoh S-H, et al. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomedical Materials, 2008, 3(3): 034108
Willnecker J. Novel approaches towards primate toxicology. In: Weinbauer G F, Vogel F, eds. Novel Approaches towards Primate Toxicology. Münster: Waxmann, 2006, 13–19
An Y H. Mechanical properties of bone. In: An Y H, Draughn R A, eds. Mechanical Testing of Bone and the Bone-Implant Interface. CRC Press, 1999, 41–63
Mutlu I, Oktay E. Biocompatibility of 17–4 PH stainless steel foam for implant applications. Bio-Medical Materials and Engineering, 2011, 21(4): 223–233
Li J P, Li S H, Van Blitterswijk C A, et al. Cancellous bone from porous Ti6Al4V by multiple coating technique. Journal of Materials Science: Materials in Medicine, 2006, 17(2): 179–185
Dall’Ara E, Karl C, Mazza G, et al. Tissue properties of the human vertebral body sub-structures evaluated by means of microindentation. Journal of the Mechanical Behavior of Biomedical Materials, 2013, 25: 23–32
Bosisio M R, Talmant M, Skalli W, et al. Apparent Young’s modulus of human radius using inverse finite-element method. Journal of Biomechanics, 2007, 40(9): 2022–2028
Zhao M, An M, Wang Q, et al. Quantitative proteomic analysis of human osteoblast-like MG-63 cells in response to bioinert implant material titanium and polyetheretherketone. Journal of Proteomics, 2012, 75(12): 3560–3573
Pan J, Wang Y, Qin S, et al. Grafting reaction of poly(D,L) lactic acid with maleic anhydride and hexanediamine to introduce more reactive groups in its bulk. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2005, 74B(1): 476–480
Meyers S R, Khoo X, Huang X, et al. The development of peptidebased interfacial biomaterials for generating biological functionality on the surface of bioinert materials. Biomaterials, 2009, 30(3): 277–286
Dunne C F, Twomey B, O’Neill L, et al. Co-blasting of titanium surfaces with an abrasive and hydroxyapatite to produce bioactive coatings: substrate and coating characterisation. Journal of Biomaterials Applications, 2014, 28(5): 767–778
Jun S H, Lee E J, Jang T S, et al. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. Journal of Materials Science: Materials in Medicine, 2013, 24(3): 773–782
Grinet M A V M, Zanin H, Campos Granato A E, et al. Fast preparation of free-standing nanohydroxyapatite-vertically aligned carbon nanotube scaffolds. Journal of Materials Chemistry B, 2014, 2(9): 1196–1204
Reed M J, Damodarasamy M, Chan C K, et al. Cleavage of hyaluronan is impaired in aged dermal wounds. Matrix Biology, 2013, 32(1): 45–51
Del Buono A, Chan O, Maffulli N. Achilles tendon: functional anatomy and novel emerging models of imaging classification. International Orthopaedics, 2013, 37(4): 715–721
Domaschke H, Gelinsky M, Burmeister B, et al. In vitro ossification and remodeling of mineralized collagen I scaffolds. Tissue Engineering, 2006, 12(4): 949–958
Wang Y, Cui F Z, Hu K, et al. Bone regeneration by using scaffold based on mineralized recombinant collagen. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2008, 86B(1): 29–35
Liao S S, Cui F Z. In vitro and in vivo degradation of mineralized collagen-based composite scaffold: nanohydroxyapatite/collagen/poly(L-lactide). Tissue Engineering, 2004, 10(1–2): 73–80
Zhang X, Guo WG, Cui H, et al. In vitro and in vivo enhancement of osteogenic capacity in a synthetic BMP-2 derived peptidecoated mineralized collagen composite. Journal of Tissue Engineering and Regenerative Medicine, 2014, doi: 10.1002/term.1705
Zeugolis D I, Li B, Lareu R R, et al. Collagen solubility testing, a quality assurance step for reproducible electro-spun nano-fibre fabrication. A technical note. Journal of Biomaterials Science: Polymer Edition, 2008, 19(10): 1307–1317
Maroudas A I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature, 1976, 260(5554): 808–809
Elden H R. Rate of swelling of collagen. Science, 1958, 128(3339): 1624–1625
Gupta A, Tripathi G, Lahiri D, et al. Compression molded ultra high molecular weight polyethylene-hydroxyapatite-aluminum oxide-carbon nanotube hybrid composites for hard tissue replacement. Journal of Materials Science and Technology, 2013, 29(6): 514–522
Zhang Z, Li G, Shi B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. Journal of the Society of Leather Technologists and Chemists, 2006, 90(1): 23–28
Li Y, Li Y, Du Z, et al. Comparison of dynamic denaturation temperature of collagen with its static denaturation temperature and the configuration characteristics in collagen denaturation processes. Thermochimica Acta, 2008, 469(1–2): 71–76
Navarro M, Michiardi A, Castaño O, et al. Biomaterials in orthopaedics. Journal of the Royal Society: Interface, 2008, 5(27): 1137–1158
Johnson K A. Wolff’s law continues to inspire orthopaedic research. Veterinary and Comparative Orthopaedics and Traumatology, 2014, 27(1): V–VI
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, ZY., Tao, CS., Cui, H. et al. High-strength mineralized collagen artificial bone. Front. Mater. Sci. 8, 53–62 (2014). https://doi.org/10.1007/s11706-014-0237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-014-0237-9