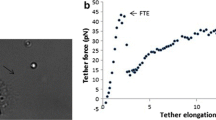
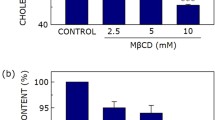
Abstract
Cholesterol plays a significant role in the organization of lipids and modulation of membrane dynamics in mammalian cells. However, the effect of cholesterol depletion on the eukaryotic cell membranes seems controversial. In this study, the effects of cholesterol on the topography and mechanical behaviors of CHO-K1 cells with manipulated membrane cholesterol contents were investigated by atomic force microscopy (AFM) technique. Here, we found that the depletion of cholesterol in cell membranes could increase the membrane stiffness, reduce the cell height as well as promote cell retraction and detachment from the surface, whereas the cholesterol restoration could reverse the effect of cholesterol depletion on the membrane stiffness. Increased methyl-β-cyclodextrin levels and incubation time could significantly increase Young’s modulus and degree of stiffing on cell membrane and cytoskeleton. This research demonstratede importance of cholesterol in regulating the dynamics of cytoskeleton-mediated processes. AFM technique offers excellent advantages in the dynamic monitoring of the change in membranes mechanical properties and behaviors during the imaging process. This promising technology can be utilized in studying the membrane properties and elucidating the underlying mechanism of distinct cells in the near-native environment.

Similar content being viewed by others
References
de Oliveira Andrade L. Understanding the role of cholesterol in cellular biomechanics and regulation of vesicular trafficking: The power of imaging. Biomedical Spectroscopy and Imaging, 2016, 5 (s1): S101–S117
Evangelisti E, Cecchi C, Cascella R, Sgromo C, Becatti M, Dobson C M, Chiti F, Stefani M. Membrane lipid composition and its physicochemical properties define cell vulnerability to aberrant protein oligomers. Journal of Cell Science, 2012, 125(10): 2416–2427
Redondo-Morata L, Giannotti M I, Sanz F. Influence of cholesterol on the phase transition of lipid bilayers: A temperature-controlled force spectroscopy study. Langmuir, 2012, 28(35): 12851–12860
Zhao L, Temelli F. Preparation of liposomes using supercritical carbon dioxide via depressurization of the supercritical phase. Journal of Food Engineering, 2015, 158: 104–112
Magarkar A, Dhawan V, Kallinteri P, Viitala T, Elmowafy M, Rog T, Bunker A. Cholesterol level affects surface charge of lipid membranes in saline solution. Scientific Reports, 2014, 4: 2045–2322
Zhao L, Temelli F, Curtis J M, Chen L. Encapsulation of lutein in liposomes using supercritical carbon dioxide. Food Research International, 2017, 100: 168–179
de Meyer F, Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(10): 3654–3658
Sułkowski W, Pentak D, Nowak K, Sułkowska A. The influence of temperature, cholesterol content and pH on liposome stability. Journal of Molecular Structure, 2005, 744: 737–747
Zhao L, Temelli F, Curtis J M, Chen L. Preparation of liposomes using supercritical carbon dioxide technology: Effects of phospholipids and sterols. Food Research International, 2015, 77: 63–72
Zhao L, Temelli F. Preparation of liposomes using a modified supercritical process via depressurization of liquid phase. Journal of Supercritical Fluids, 2015, 100: 110–120
Khatibzadeh N, Spector A A, Brownell W E, Anvari B. Effects of plasma membrane cholesterol level and cytoskeleton F-actin on cell protrusion mechanics. PLoS One, 2013, 8(2): e57147
Mañes S, Martínez-A C. Cholesterol domains regulate the actin cytoskeleton at the leading edge of moving cells. Trends in Cell Biology, 2004, 14(6): 275–278
Sun M, Northup N, Marga F, Huber T, Byfield F J, Levitan I, Forgacs G. The effect of cellular cholesterol on membranecytoskeleton adhesion. Journal of Cell Science, 2007, 120(13): 2223–2231
Norman L L, Oetama R J, Dembo M, Byfield F, Hammer D A, Levitan I, Aranda-Espinoza H. Modification of cellular cholesterol content affects traction force, adhesion and cell spreading. Cellular and Molecular Bioengineering, 2010, 3(2): 151–162
Yang Y T, Liao J D, Lin C C K, Chang C T, Wang S H, Ju M S. Characterization of cholesterol-depleted or-restored cell membranes by depth-sensing nano-indentation. Soft Matter, 2012, 8(3): 682–687
Kilbride P, Woodward H J, Tan K B, Thanh N T, Chu K E, Minogue S, Waugh MG. Modeling the effects of cyclodextrin on intracellular membrane vesicles from Cos-7 cells prepared by sonication and carbonate treatment. PeerJ, 2015, 3: e1351
Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochimica et Biophysica Acta (BBA)—Biomembranes, 2007, 1768(6): 1311–1324
Christian A, Haynes M, Phillips M, Rothblat G. Use of cyclodextrins for manipulating cellular cholesterol content. Journal of Lipid Research, 1997, 38(11): 2264–2272
Romanenko V G, Fang Y, Byfield F, Travis A J, Vandenberg C A, Rothblat G H, Levitan I. Cholesterol sensitivity and lipid raft targeting of Kir2. 1 channels. Biophysical Journal, 2004, 87(6): 3850–3861
Mahammad S, Parmryd I. Cholesterol depletion using methylcyclodextrin. Methods in Membrane Lipids, 2015: 91–102
Romanenko V G, Rothblat G H, Levitan I. Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol. Biophysical Journal, 2002, 83(6): 3211–3222
Levitan I, Christian A E, Tulenko T N, Rothblat G H. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. Journal of General Physiology, 2000, 115(4): 405–416
Niu S L, Mitchell D C, Litman B J. Manipulation of cholesterol levels in rod disk membranes by methyl-β-cyclodextrin effects on receptor activation. Journal of Biological Chemistry, 2002, 277(23): 20139–20145
Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with β-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry, 1995, 34(42): 13784–13793
Roduit C, van der Goot F G, De Los Rios P, Yersin A, Steiner P, Dietler G, Catsicas S, Lafont F, Kasas S. Elastic membrane heterogeneity of living cells revealed by stiff nanoscale membrane domains. Biophysical Journal, 2008, 94(4): 1521–1532
Bronder A M, Bieker A, Elter S, Etzkorn M, Häussinger D, Oesterhelt F. Oriented membrane protein reconstitution into tethered lipid membranes for AFM force spectroscopy. Biophysical Journal, 2016, 111(9): 1925–1934
Casuso I, Khao J, Chami M, Paul-Gilloteaux P, Husain M, Duneau J P, Stahlberg H, Sturgis J N, Scheuring S. Characterization of the motion of membrane proteins using high-speed atomic force microscopy. Nature Nanotechnology, 2012, 7(8): 525–529
Hutter J L, Bechhoefer J. Calibration of atomic-force microscope tips. Review of Scientific Instruments, 1993, 64(7): 1868–1873
Sneddon I N. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. International Journal of Engineering Science, 1965, 3(1): 47–57
Matzke R, Jacobson K, Radmacher M. Direct, high-resolution measurement of furrow stiffening during division of adherent cells. Nature Cell Biology, 2001, 3(6): 607–610
Emad A, Heinz W F, Antonik M D, D’Costa N P, Nageswaran S, Schoenenberger C A, Hoh J H. Relative microelastic mapping of living cells by atomic force microscopy. Biophysical Journal, 1998, 74(3): 1564–1578
Lam R S, Shaw A R, Duszyk M. Membrane cholesterol content modulates activation of BK channels in colonic epithelia. Biochimica et Biophysica Acta (BBA)—Biomembranes, 2004, 1667(2): 241–248
Toselli M, Biella G, Taglietti V, Cazzaniga E, Parenti M. Caveolin-1 expression and membrane cholesterol content modulate N-type calcium channel activity in NG108-15 cells. Biophysical Journal, 2005, 89(4): 2443–2457
Oh H, Mohler E R III, Tian A, Baumgart T, Diamond S L. Membrane cholesterol is a biomechanical regulator of neutrophil adhesion. Arteriosclerosis, Thrombosis, and Vascular Biology, 2009, 29(9): 1290–1297
Corvera S, DiBonaventura C, Shpetner H S. Cell confluencedependent remodeling of endothelial membranes mediated by cholesterol. Journal of Biological Chemistry, 2000, 275(40): 31414–31421
Frankel D, Pfeiffer J, Surviladze Z, Johnson A, Oliver J, Wilson B, Burns A. Revealing the topography of cellular membrane domains by combined atomic force microscopy/fluorescence imaging. Biophysical Journal, 2006, 90(7): 2404–2413
Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz M P, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(24): 13964–13969
Byfield F J, Tikku S, Rothblat G H, Gooch K J, Levitan I. OxLDL increases endothelial stiffness, force generation, and network formation. Journal of Lipid Research, 2006, 47(4): 715–723
Zhang X, Hurng J, Rateri D L, Daugherty A, Schmid-Schönbein G W, Shin H Y. Membrane cholesterol modulates the fluid shear stress response of polymorphonuclear leukocytes via its effects on membrane fluidity. American Journal of Physiology. Cell Physiology, 2011, 301(2): C451–C460
Borbiev T, Radel C, Rizzo V. Participation of caveolae in β1 integrin-mediated mechanotransduction. FASEB Journal, 2007, 21 (6): A752–A752
Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nature Communications, 2015, 6(1): 8512
Byfield F J, Aranda-Espinoza H, Romanenko V G, Rothblat G H, Levitan I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophysical Journal, 2004, 87(5): 3336–3343
Hissa B, Pontes B, Roma P M S, Alves A P, Rocha C D, Valverde T M, Aguiar P H N, Almeida F P, Guimaraes A J, Guatimosim C, et al. Membrane cholesterol removal changes mechanical properties of cells and induces secretion of a specific pool of lysosomes. PLoS One, 2013, 8(12): e82988
Brown R E. Sphingolipid organization in biomembranes: What physical studies of model membranes reveal. Journal of Cell Science, 1998, 111(1): 1–9
Pralle A, Keller P, Florin E L, Simons K, Hörber J H. Sphingolipidcholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. Journal of Cell Biology, 2000, 148(5): 997–1008
Wakatsuki T, Schwab B, Thompson N C, Elson E L. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. Journal of Cell Science, 2001, 114(5): 1025–1036
Khatibzadeh N, Gupta S, Farrell B, Brownell WE, Anvari B. Effects of cholesterol on nano-mechanical properties of the living cell plasma membrane. Soft Matter, 2012, 8(32): 8350–8360
Zhang L, Bennett W F D, Zheng T, Ouyang P K, Ouyang X P, Qiu X Q, Luo A Q, Karttunen M, Chen P. Effect of cholesterol on cellular uptake of cancer drugs pirarubicin and ellipticine. Journal of Physical Chemistry B, 2016, 120(12): 3148–3156
Ramprasad O, Srinivas G, Rao K S, Joshi P, Thiery J P, Dufour S, Pande G. Changes in cholesterol levels in the plasma membrane modulate cell signaling and regulate cell adhesion and migration on fibronectin. Cytoskeleton, 2007, 64(3): 199–216
López C A, de Vries A H, Marrink S J. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Computational Biology, 2011, 7(3): e1002020
Acknowledgements
The authors sincerely appreciate the financial support by the Natural Sciences and Engineering Research Council of Canada Discovery Grants Program as well as the Program of Scientific Innovation Research of College Graduates in Jiangsu Province (No. CXZZ13_0455). The authors also thank Prof. Zhaobing Gao in the Shanghai Institute of Materia Medica of Chinese Academy of Science for providing the Biocatalyst AFM and related supports.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11705_2018_1775_MOESM1_ESM.pdf
Insight into the role of cholesterol in modulation of morphology and mechanical properties of CHO-K1 cells: An in situ AFM study
Rights and permissions
About this article
Cite this article
Zhang, L., Zhao, L., Ouyang, PK. et al. Insight into the role of cholesterol in modulation of morphology and mechanical properties of CHO-K1 cells: An in situ AFM study. Front. Chem. Sci. Eng. 13, 98–107 (2019). https://doi.org/10.1007/s11705-018-1775-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-018-1775-y