Abstract
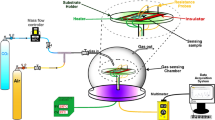
Carbon monoxide is a poisonous and hazardous gas and sensitive sensor devices are needed to prevent humans from being poisoned by this gas. A CO gas sensor has been prepared from WO3 synthesized by a sol-gel method. The sensor chip was prepared by a spin-coating technique which deposited a thin film of WO3 on an alumina substrate. The chip samples were then calcined at 300, 400, 500 or 600 °C for 1 h. The sensitivities of the different sensor chips for CO gas were determined by comparing the changes in electrical resistance in the absence and presence of 50 ppm of CO gas at 200 °C. The WO3 calcined at 500 °C had the highest sensitivity. The sensitivity of this sensor was also measured at CO concentrations of 100 ppm and 200 ppm and at operating temperatures of 30 and 100 °C. Thermogravimetric analysis of the WO3 calcined at 500 °C indicated that this sample had the highest gas adsorption capacity. This preliminary research has shown that WO3 can serve as a CO gas sensor and that is should be further explored and developed.
Similar content being viewed by others
References
Wang S H, Chou T C, Liu C C. Nano-crystalline tungsten oxide NO2 sensor. Sensors and Actuators. B, Chemical, 2003, 94(3): 343–351
Liu Z, Miyauchi M, Yamazaki T, Zhen Y. Facile synthesis and NO2 gas sensing of tungsten oxide nanorods assembled microspheres. Sensors and Actuators. B, Chemical, 2009, 140(2): 514–519
Boulova M, Gaskov A, Lucazeau G. Tungsten oxide reactivity versus CH4, CO and NO2 molecules studied by Raman spectroscopy. Sensors and Actuators. B, Chemical, 2001, 81(1): 99–106
Yan A, Xie C, Zeng D, Cai S, Hu M. Synthesis, formation mechanism and sensing properties of WO3 hydrate nanowire nettedspheres. Materials Research Bulletin, 2010, 45(10): 1541–1547
Kanan S M, Tripp C P. Synthesis, FTIR studies and sensor properties of WO3 powders. Current Opinion in Solid State and Materials Science, 2007, 11(1–2): 19–27
Su X, Li Y, Jian J, Wang J. In situ etching WO3 nanoplates: Hydrothermal synthesis, photoluminescence and gas sensor properties. Materials Research Bulletin, 2010, 45(12): 1960–1963
Deepa M, Singh P, Sharma S N, Agnihotry S A. Effect of humidity on structure and electrochromic properties of sol-gel-derived tungsten oxide films. Solar Energy Materials and Solar Cells, 2006, 90(16): 2665–2682
Ozkan E, Lee S H, Liu P, Tracy C E, Tepehan F Z, Pitts J R, Deb S K. Electrochromic and optical properties of mesoporous tungsten oxide films. Solid State Ionics, 2002, 149(1–2): 139–146
Pyper O, Schollhorn R, Donkers J J T M, Krings L H M. Nanocrystalline structure of WO3 thin Films prepared by the sol-gel technique. Materials Research Bulletin, 1998, 33(7): 1095–1101
Su L, Lu Z. All solid-state smart window of electrodeposited WO3 and TiO2 particulate film with PTREFG gel electrolyte. Journal of Physics and Chemistry of Solids, 1998, 59(8): 1175–1180
Chang K H, Hu C C, Huang C M, Liu Y L, Chang C I. Microwaveassisted hydrothermal synthesis of crystalline WO3-WO3·0.5H2O mixtures for pseudocapacitors of the asymmetric type. Journal of Power Sources, 2011, 196(4): 2387–2392
Gillet M, Masek K, Gillet E. Structure of tungsten oxide nanoclusters. Surface Science, 2004, 566–568: 383–389
Hidayat D, Purwanto A, Wang W N, Okuyama K. Preparation of size-controlled tungsten oxide nanoparticles and evaluation of their adsorption performance. Materials Research Bulletin, 2010, 45(2): 165–173
Ha J H, Muralidharan P, Kim D K. Hydrothermal synthesis and characterization of self-assembled h-WO3 nanowires/nanorods using EDTA salts. Journal of Alloys and Compounds, 2009, 475(1–2): 446–451
Ramana C V, Utsunomiya S, Ewing R C, Julien C M, Becker U. Structural stability and phase transitions in WO3 thin films. Journal of Physical Chemistry B, 2006, 110(21): 10430–10435
Houx N L, Pourroy G, Camerel F, Comet M, Spitzer D. WO3 nanoparticles in the 5–30 nm range by solvothermal synthesis under microwave or resistive heating. Journal of Physical Chemistry B, 2010, 114: 155–161
Yous B, Robin S, Donnadieu A. Chemical vapor deposition of tungsten oxides: A comparative study by XPS, XRD and RHEED. Materials Research Bulletin, 1984, 19: 1349–1354
Pyun S I, Kim D J, Bae J S. Hydrogen transport through r.f. magnetron sputtered amorphous and crystalline WO3 films. Journal of Alloys and Compounds, 1996, 244(1–2): 16–22
Deki S, Beleke A B, Kotani Y, Mizuhata M. Synthesis of tungsten oxide thin film by liquid phase deposition. Materials Chemistry and Physics, 2010, 123(2–3): 614–619
Abdullah S F, Radiman S, Hamid M A A, Ibrahim N B. Effect of calcinations temperature on the surface morphology and crystallinity of tungsten (VI) oxide nanorods prepared using colloidal gas aphrons method. Colloids and Surfaces A, 2006, 280: 88–94
Brinker C J, Scherer G W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. San Diego, USA: Academic Press. Inc., 1990, 2–6
Ho G W. Gas sensor with nanostructured oxide semiconductor materials. Science of Advanced Materials, 2011, 3(2): 150–168
Susanti D, Nugroho S H, Nisfu H, Nugroho E P, Purwaningsih H, Kusuma G E, Shih S J. Comparative analysis of WO3 nanomaterial synthesized using a sol-gel method followed by calcination and hydrothermal treatments. Frontiers of Chemical Science and Engineering, 2012, 6(4): 371–380
Cullity B D, Stock S R. Elements of X-Ray Diffraction. 3rd ed. New Jersey, USA: Prentice Hall, 2001, 170–172
Szilágyi I M, Madarász J, Pokol G, Király P, Tárkányi G, Saukko S, Mizsei J, Tòth A L, Szabò A, Varga-Josepovits K. Stability and controlled composition of hexagonal WO3. Chemistry of Materials, 2008, 20(12): 4116–4125
Daniel M F, Desbat B, Lassegues J C, Gerard B, Figlarz M. Infrared and Raman study of WO3 tungsten trioxide and WO3·xH2O tungsten trioxide hydrate. Journal of Solid State Chemistry, 1987, 67(2): 235–247
Tamaki J, Zhang Z, Fujimori K, Akiyama M, Harada T, Miura N, Yamazoe N. Grain-size effects in tungsten oxide-based sensor for nitrogen oxides. Journal of the Electrochemical Society, 1994, 141(8): 2207–2210
Kocemba I, Rynkowski J. The influence of catalytic activity on the response of Pt/SnO2 gas sensors to carbon monoxide and hydrogen. Sensors and Actuators. B, Chemical, 2011, 155(2): 659–666
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Susanti, D., Diputra, A.A.G.P., Tananta, L. et al. WO3 nanomaterials synthesized via a sol-gel method and calcination for use as a CO gas sensor. Front. Chem. Sci. Eng. 8, 179–187 (2014). https://doi.org/10.1007/s11705-014-1431-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-014-1431-0