Abstract
Main aim of this study is to assess the effect of a structured, interdisciplinary, surgical, team-training protocol in robotic gynecologic surgery, with the gradual integration of an advanced nurse practitioner. Data from all robotic surgical procedures were prospectively acquired. The surgical team consisted of one experienced surgeon and two surgical fellows and the scrub nurse team from three advance nurse practitioners, specialized in robotic surgery. The training was performed in a four-phase manner over 4 years and included theoretical training, hands-on training and team-communication skills enhancement. Scrub nurses increasingly adopted an active role during surgery. For a period of 4 years, 175 patients could be included in the analysis. All of them underwent a robotic gynecologic procedure. Mean docking time decreased from 45.3 to 27.3 min (p < 0.001), mean operating time from 235 to 179 min (p = 0.0071) and costs per case from 17,891 to 14,731 Swiss Francs (p = 0.035). There were no statistically significant changes in perioperative complications and conversions to laparotomy. An interdisciplinary long-term training protocol for high specialized robotic surgery within a “fixed” team with the gradually addition of an advanced study nurse improves the efficacy of the procedure in terms of time and costs. Although the surgery is performed quicker, the same performance and quality of surgical care could be reached.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robotic-assisted laparoscopic surgery is the latest major development in minimally invasive gynecologic surgery. The Da Vinci robotic system was developed by Intuitive Surgical and was approved for gynecologic surgery by the United States Federal Drug Administration (FDA) in April 2005 [1]. Many advanced laparoscopic surgical interventions that would have been otherwise performed with an abdominal incision are now being performed with minimally invasive techniques utilizing this system [2].
Robotic surgery seems to have a steeper learning curve than conventional laparoscopy and demonstrates a faster adoption rate [3, 4]. It has been estimated that the overall proportion of cases performed through minimally invasive surgery has increased from 9 to 36% in the third year after introducing the robot and there is evidence that during the period 2013–2014 over 50% of all hysterectomies for benign indications in the United States were performed with the robot [5, 6]. Among others, this has been attributed to the fact that the robotic approach provides similar outcomes to conventional laparoscopy in terms of patient safety and efficacy while minimizing morbidity, postoperative pain, blood loss and hospital stay [7].
However, after an initial period of enthusiastic reception and commercial increment, robotic surgery has often been a target for criticism, mostly due to the long operating time and associated health costs [8]. Published data suggests that the costs of robotic-assisted hysterectomies can be up to 1.5–3 times higher than the costs of conventional laparoscopic techniques, despite overall shorter hospital stay and lower conversion rates [9]. This assumption, however, derives either from retrospective data or from publications, which did not provide appropriate economic evaluation (such as cost-minimization analysis, cost-effectiveness analysis, cost–benefit analysis and cost–utility analysis) [10, 11].
Structured and continuous training of young surgeons and surgeons with no robotic experience is crucial for the incorporation of robotic methods in clinical practice and maintenance of expertise, but it has also led to a re-thinking of training and teaching methods, not only for surgeons but for the entire operating room team too, including scrub and circulating nurses [12, 13]. Published studies have shown the advantages of highly trained personnel in the operating room but also the challenges and special requirements of continuous and state-of-the-art, multidisciplinary training in the operating room [14]. Also, since robotic surgery costs are generated in the operating room, there is evidence that robotic training and gain of surgical experience and expertise within the surgical team can lead to significantly shorter operating times and thus to eventually lower costs, even when compared to conventional techniques [15, 16].
Aim of this prospective study is to assess the impact and feasibility of a structured robotic surgery training protocol for gynecologic surgeons with the active integration of an Advanced Nurse Practitioner (ANP) and to present our experience in a university hospital setting.
Materials and methods
This study, which obtained the approval of the local ethical committee (ID 355/11), was performed in the Department of Gynecology and Gynecologic Oncology of the University Hospital of Basel, and is a part of a multidisciplinary project to assess and support the implementation of robotic surgery as well as the employment of Advanced Nurse Practitioners, who are strategically employed in sensitive positions, to promote quality and patients’ safety. Our department began with the use of the four-arm da Vinci Si robot (da Vinci Surgical System ©, Intuitive Surgical Inc., Sunnyvale, CA, USA) on January 2012 for cases of gynecologic malignant and benign disease. A gynecologic/oncologic surgeon, with long-term experience in robotic surgery (VH) initiated the use of the robotic system. After the first period of acquaintance with the team and the setting, it was decided to begin with the implementation of the training protocol to expand and support the use of the system.
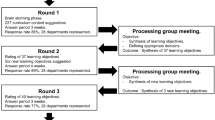
Aim of our training protocol aimed was the long-term, gradual reduction of the number of surgeons while simultaneously taking advantage of the acquired experience of the staff in robotic surgery. Hence, the implementation of the protocol corresponds to the outset of robotic surgery in our department. Since there was no previous experience with robotic surgery within the team, we decided to conduct a four-step-approach, which can be summarized as follows (see also Fig. 1):
Step 1 (baseline, time group 1): Initiation with a gynecologic/oncologic surgeon, with long experience in robotic surgery. Other team-members with no experience in robotic surgery. Random assigned first assistance as well as scrub and circulating nurse.
Step 2 (time group 2): Addition of two robotic surgery fellows (TK, CM) to the team, who alternately provided first assistance to the experienced surgeon. The two fellows received throughout a rigorous and continuous training including theoretical training, hands-on workshops and surgical training in robotic training centers in Europe as well as in-team feedback for their performance and expectations. The two fellows started having console time, starting with small tasks and gradually taking over whole steps of the surgical procedure. Scrub and circulating nurses were still randomly assigned.
Step 3 (time group 3): The two fellows are now fully integrated as surgeons to the team and the three surgeons were alternately performing, with one other providing first assistance. Thus, at any given time two experienced surgeons were present. Additionally, the scrub nurse team was reduced to three ‘fixed’ team members, who alternated places in preparing the robotic arms, performing intraoperative corrections and handing surgical instruments. Simultaneously, they received continuous theoretical and hands-on training with the surgeons. Gradually, integration of the scrub nurse as the first assistant, including instrumentation, suction, real-time correction of the robotic arms and active suturing assistance.
Step 4 (time group 4): Three surgeons (same as step 3). Addition of two ANP, who, alternately, completely took over the first assistance. At any given time only one out of three surgeons and one out of two ANP were by the patient. ANP conducts preparation, controlling and corrections of the robotic system and has full responsibility for the execution of the tasks at the patients while the surgeon sits at the console.
During this time, the entire team (surgeons and nurses) received continuous education and training on robotic surgery. The programme included three main educational domains:
-
1.
theoretical training on laparoscopic surgery and operating room settings (anatomy, functional characteristics of the robot, regular literature updates),
-
2.
intensive hands-on training (courses on robotic surgery, international workshops, simulation of emergency scenarios, training on cadaver and pelvic models) and
-
3.
communications training (team time-out and sign-out, regular meetings, de-briefings and feedback after difficult situations, mutual decisions for surgery planning, joint social activities).
We collected data of all patients who underwent robotic surgery for malignant and benign gynecologic disease between January 2012 and December 2015. The operating time was defined as the time between skin incision and skin closure (at the trocar area), whereas the docking time was defined as the time between the first skin incision and console start with the surgeon in place and operating the robot. All procedures were performed according to our intern clinical practice protocols and in accordance with national recommendations, whereas especially in oncologic patients, the international requirements of clinical practice in oncology were applied. Patients with the diagnosis ovarian cancer in our analysis did not include the first diagnosis of ovarian, since in these cases the primary procedure is laparotomy, but it refers to patients who received hysterectomy or lymph node excision after the initial diagnosis was made.
This study was per protocol designed to conduct an analysis of perioperative data (such as docking and operating time) adverse events and costs, which were generated in the operating room and during the hospital stay, excluding costs from readmissions or outpatient treatments. Data was retrieved prospectively with the assistance of the hospital’s administrative and financial department.
Statistical analysis
Descriptive statistics are presented as counts and frequencies for categorical data and median [interquantile range] for metric variables. Overall p values correspond to Kruskall–Wallis test (for median) and chi-squared or exact Fisher’s test when the expected frequencies is less than 5 in some cell.
To compare docking time, operative time, console time, overall costs, op costs, linear regression was performed between time groups. Results are presented as differences of means with corresponding 95% confidence intervals and p values.
To compare time groups between a hospital stay and blood loss, Dunn’s test was performed and 95% confidence intervals of the differences of medians were presented [17]. Confidence intervals were based on bootstrap. Dunn's test was performed because data distributions are considered as skewed. p values were considered as exploratory and not adjusted for multiple comparisons. A p value < 0.05 is considered as significant.
All evaluations were done using the statistical software R v 3.6.1 [18].
Results
We could include 175 robotic laparoscopic procedures, which were performed in the department of gynecology and gynecologic oncology between January 2012 and December 2015. Table 1 shows number of patients included in each step as well as patients’ characteristics and main indications. Between time group 1 and time group 4, mean docking time decreased significantly from 45.3 to 27.3 min (mean difference: 18; CI 12–24 p < 0.001), mean operative time decreased from 235 to 179 min (mean difference: 57; CI 16–98; p = 0.0071. The mean costs per case generated in the operating room were reduced from 6907 to 6506 (mean difference: 402; CI − 758 to 1562; p = 0.50) but were not significant. The mean overall costs per case decreased significantly, from 17,891 to 14,731 Swiss Francs (mean difference: 3159; CI 213–6105; p = 0.035). Median hospital stay decreased not significantly from 4 to 3 days (median difference: 1; CI 0–2 p = 0.14). There were no statistically significant changes in perioperative complications and conversions to laparotomy (8.11% vs. 3.00%, p = 0.457 and 8.11% vs. 5.88%, p = 0.390, respectively). Also, there were no statistically significant differences in intraoperative complications and conversions to laparotomy. Overall median blood loss was significantly higher at the end of the study period (100 ml vs. 150 ml, median difference: − 50, CI − 123 to 25, p = 0.012) (for detailed information please see Table 2, as well as Fig. 2a–c).
Discussion
This prospective study presents the impact of a structured educational protocol on the effectiveness of a team of both young surgeons and specialized nurses. To our knowledge, this is the first prospective analysis of such an effort, which includes the education of medical doctors as surgical fellows and simultaneously the introduction of the Advanced Nurse Practitioner as constant team members in robotic surgery, with an active role during the surgical intervention. Our strategic goal was to increase the number of experienced robotic surgeons in our team, without compromising quality and patients’ care, since the growing demand on minimally invasive surgery and novel technologies dictated the investment on and the further development of robotic surgery in gynecological patients of our department.
The importance of continuous surgical training and frequent surgical activity in the operating room in achieving and maintaining surgical excellence and improving perioperative patients’ care is undeniable [19, 20]. In addition, education and training of young surgeons in an out of the operating room is crucial for gaining surgical proficiency, mostly so in complex interventions [21]. A systematic review of Miskovic et al. reported that trainees could obtain clinical results similar to those of expert surgeons in laparoscopic colorectal surgery if mentored and supervised by an experienced trainer [22]. They showed that a structured training of young surgeons on laparoscopic colorectal intervention with a mentor assignment, results in similar conversion rates (p = 0.28), complications (p = 0.49), anastomosis leak (p = 0.36) and mortality (p = 0.56), when compared to interventions performed by experts. However, there is evidence that due to variable factors, such as novel non-interventional therapies and the expansion of radiological-interventional methods, residents and trainees can suffer from the lack of proper surgical training, much more so in advanced and not-so-frequent performed surgical interventions [23].
Robotic surgery can be definitely seen as a ‘special case’ as far as surgical training is concerned. The vast majority of surgical interventions are performed with one console and there is one principal surgeon, controlling the robotic arms and the camera. The physical distance between the operating surgeon and operative field creates a barrier that prevents a resident learner from appreciating how an attending's physical movements directly translate into the simultaneous tissue manipulation observed on the screen [24]. The learner often struggles to recreate these movements, while other physical barriers, such as screen size, arm placement and the lack of a haptic feedback may compromise the young surgeon’s experience and also decrease the trainers consent to give over control [25].
With the increasing demand and use of robotic systems, residents’ and OR team training became profoundly important for patients’ safety and quality assurance. The development of a structured training curriculum in robotic surgery has been proposed as the major prerequisite for the successful transfer of skills to young surgeons from their experienced mentors [26]. As mentioned before, this is not an easy task in the case of robotic surgery, but there is evidence that in a proper setting, the training of young surgeons can be feasible, efficient and safe [27].
Similarly important, although not as thoroughly examined, seems to be the training of the operating room nurse. Similar to medical trainees, nurses who work with robotic systems, are confronted with a number of conceptual and technical challenges. First, two of the most important behavioral markers for successful nursing in the operating room, namely the eye gaze/contact with the surgeon and the anticipation movements, are compromised, due to the fact, that the surgeon sits alone at the console [28]. Second, robotic surgery demands high technical competence and more active role from the operating room nurse, whose responsibilities involve helping the surgeon, paying attention to the rules of asepsis by distinguishing the sterile and nonsterile parts of the robot, placing the robot arms, reading the data received from the videoscopic screen correctly and quickly, reporting to the surgeon and taking immediate measures in case of possible power failure [29, 30]. Consequently, these demands require a comprehensive and effective training of the robotic nurses, to maintain quality but also to overcome individual fear and hesitation when dealing with the robotic system [31].
It can be argued that the conventional model of a surgical curriculum is mainly single surgeon oriented and hierarchical structured, lacking interdisciplinary involvement, rather impersonal and mostly short-term regarding team- and professional development of the people involved. The approach in this study focused on interprofessional interaction and awareness, team strengthening measures, continuous medical and technical training and long-term planning and objective targeting.
Of course, the improved outcomes could partially be explained through the gained experience of the surgeon and simply the comfort of all participants with the robotic technique and there is evidence that surgical team training, including scrub and circulating nurses can improve outcomes [32]. Our data, being to our knowledge the first publication which includes the active integration of an advanced nurse practitioner in the role of the first assistant, show that a structured training protocol based on the increasingly active role of operating room nurses and novice fellow-surgeons is not only feasible but also beneficial in terms of patients’ safety, perioperative time and generated costs. Outcomes like improved docking time, which continuously decreased while performed by different team members throughout the different phases, support the fact that team training and active involvement of everyone present in the operating room, and not only the main surgeon, is crucial. This could be shown in a prospective manner, including all gynecologic patients who were treated with robotic surgery. Our results also showed an increase in blood loss between the first and the last training phase. Blood loss estimation in our study was performed empirically through the surgical team, not on the basis of absolute quantitative methods, such as fluid balance in the suction device or gauze weighing. This fact alone could present a strong bias for the estimated blood loss. Additionally, in step 4—in contrast to step 3—the first assistance was performed solely through the ANP, so there might have been a significant variability regarding suction, irrigation, etc. which could affect the estimation of blood loss. However, the small difference in the mean blood loss (50 ml) is not of clinical significance. This is also indicated from the not significantly different complications rate, including the need for blood transfusion.
Main limitations of this study include the lack of a control group and the fact that our starting point was very early in the adoption process of robotic surgery in our institution, which could be factors that limit the generalization of the results. Further limitations of this study is the small sample size (mostly in phase 1 and 4) as well as the lack of a power analysis.
Conclusion
Although often criticized, robotic surgery is still gaining popularity and the number of robotic procedures is rising. Not only medical but also nursing personnel face important technical and conceptual challenges when using robotic surgical systems. Multidisciplinary, continuous and structured training should be offered to all teams working with robotic systems in order to maintain quality and improve skills and technical competence.
Availability of data and material
Data and source documents are available for review through the local ethics committee.
References
Weinberg L, Rao S, Escobar PF (2011) Robotic surgery in gynecology: an updated systematic review. Obstet Gynecol Int. https://doi.org/10.1155/2011/852061
Payne TN, Pitter MC (2011) Robotic-assisted surgery for the community gynecologist: can it be adopted? Clin Obstet Gynecol 54(3):391–411
Wright JD, Ananth CV, Lewin SN et al (2013) Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. J Am Med Assoc 309(7):689–698
Paley PJ, Veljovich DS, Shah CA et al (2011) Surgical out-comes in gynecologic oncology in the era of robotics: analysis of first 1000 cases. Am J Obstet Gynecol 204(6):551.e1-551.e9
Swenson CW, Kamdar NS, Harris JA, Uppal SM, Campbell DA Jr, Morgann DM (2016) Comparison of robotic and other minimally invasive routes of hysterectomy for benign indications. Am J Obstet Gynecol 215(5):650
Gaia G, Holloway RW, Santoro L, Ahmad S, Silverio E, Spinillo A (2010) Robotic-assisted hysterectomy for endometrial cancer compared with traditional laparoscopic and laparotomy approaches. Obstet Gynecol 116:1422–1423
Barbash GI, Glied SA (2010) New Technology and Health Care Costs—the case of robot-assisted surgery. N Engl J Med 701:363–368
Tapper AM, Hannola M, Zeitlin R, Isojärvi J, Sintonen H, Ikonen TS (2014) A systematic review and cost analysis of robot-assisted hysterectomy in malignant and benign conditions. Eur J Obstet Gynecol Reprod Biol 177:1–10
Turchetti G, Palla I, Pierotti F, Cuschieri A (2012) Economic evaluation of da Vinci-assisted robotic surgery:a systematic review. Surg Endosc 26:598–606
Drummond MF, O’Brien BJ, Stoddart GL, Torrance GW (1998) Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford
Gamboa AJ, Santos RT, Sargent ER et al (2009) Long term impact of a robot assisted laparoscopic prostatectomy mini fellowship training program on postgraduate urological practice patterns. J Urol 181:778–782
Thomas AA, Derboghossians A, Chang A, Karia R, Finley DS, Slezak J, Jacobsen SJ, Chien GW (2013) Impact of trainee involvement with robotic-assisted robotic prostatectomy. J Robot Surg 7:289–293
Brusco J (2012) Establishing an effective robotic surgical team. AORN J 95(5):10–11
Rowe C, Pierce M, Tecci K, Houck C, Mandell J, Retik AB, Nguyen HT (2012) A comparative direct sot analysis of pediatric urologic robot-assisted laparoscopic surgery versus open surgery: could robot-assisted surgery be less expensive? J Endourol 26:871–877
Casella DP, Fox JA, Schneck FX, Cannon GM, Ost MC (2013) Cost analysis of pediatric robot assisted and laparoscopic pyeloplasty. J Urol 189:1083–1086
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC (1993) Complications of laparoscopic chol-ecystectomy: a national survey of 4292 hospitals and ananalysis of 77,604 cases. Am J Surg 165(9–14):12
Neumayer L, Giobbie-Hurder A, Jonasson O et al (2004) Open mesh versus laparoscopic mesh repair of inguinalhernia. N Engl J Med 350:1819–1827
Toker A, Tanju S, Ziyade S et al (2008) Learning curve in videothoracoscopic thymectomy: how many operations and in which situations? Eur J Cardiothorac Surg 34(1):155–158
Miskovic D, Wyles SM, Ni M, Darzi AW, Hanna GB (2010) Systematic review on mentoring and simulation in laparoscopic colorectal surgery. Ann Surg 252:943–951
Mark AM, Thomas WB, Andrew TJ, Mary EK, Frank RL Jr (2013) Operative experience of surgery residents: trends and challenges. J Surg Educ 70(6):783–788
Green CA, Chu SN, Huang E, Chern H, O’Sullivan P (2020) Teaching in the robotic environment: use of alternative approaches to guide operative instruction. Am J Surg 219(1):191–219
Green CA, Sullivan SO, Sarin A, Chern H (2018) Microanalysis of video from a robotic surgical procedure: implications for observational learning in the robotic environment. J Robot Surg 13:449–454
Ahmed K, Khan R, Alexandre M, Lovegrove C, Abaza R, Ahlawat R et al (2015) Development of a standardised training curriculum for robotic surgery: a consensus statement from an international multidisciplinary group of experts. BJU Int 116:93–101
Honaker MD, Paton BL, Stefanidis D, Schiffern LM (2015) Can robotic surgery be done efficiently while training residents? J Surg Educ 72(3):377–380
Korkiakangas T, Weldon SM, Bezemer J, Kneebone R (2014) Nurse–surgeon object transfer: video analysis of communication and situation awareness in the operating theatre. Int J Nurs Stud 51:1195–1206
Tabor W (2007) On the cutting edge of robotic surgery. Nursing 37(2):48–50
Zender J, Thell C (2010) Developing a successful robotic surgery program in a rural hospital. AORN J 92(1):72–86
Uslu Y, Altınbaş Y, Özercan T, van Giersbergen MY (2019) The process of nurse adaptation to robotic surgery: a qualitative study. Int J Med Robot Comput Assist Surg 15:e1996
Tan SB, Pena G, Altree M, Maddern GJ (2014) Multidisciplinary team simulation for the operating theatre: a review of the literature. ANZ J Surg 84(7–8):515–522
Funding
Open Access funding provided by Universität Basel (Universitätsbibliothek Basel).
Author information
Authors and Affiliations
Contributions
FV*: manuscript writing, data collection and analysis; RE: data collection, manuscript editing; AS: statistical analysis, manuscript editing; CM: data collection, manuscript editing; MB: data collection, manuscript editing; VH: data collection, study design, manuscript editing; TK*: manuscript writing, data collection and analysis, study conception and design; *Francesco Vigo and Tilemachos Kavvadias equally contributed to the manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interests.
Ethics approval
the study was approved by the local ethics committee (Ethikkommission Nordwestschweiz ID 355/11).
Consent to participate
Written informed consent to participate was obtained from all subjects.
Consent for publication
Written informed consent for publication was obtained from all subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vigo, F., Egg, R., Schoetzau, A. et al. An interdisciplinary team-training protocol for robotic gynecologic surgery improves operating time and costs: analysis of a 4-year experience in a university hospital setting. J Robotic Surg 16, 89–96 (2022). https://doi.org/10.1007/s11701-021-01209-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-021-01209-4