Abstract
The COVID-19 pandemic led to a decrease in surgical activity to avoid nosocomial contamination. Robotic-assisted surgery safety is uncertain, since viral dissemination could be facilitated by gas environment. We assessed the impact and safety of the COVID-19 pandemic on robotic-assisted surgery. Data were collected prospectively during lockdown (March 16th–April 30th 2020) in 10 academic centres with robotic surgical activity and was compared to a reference period of similar length. After surgery, patients with suspected COVID-19 were tested by RT-PCR. During the COVID-19 lockdown we evidenced a 60% decrease in activity and a 49% decrease in oncological procedures. However, the overall proportion of oncological surgeries was significantly higher during the pandemic (p < 0.001). Thirteen (7.2%) patients had suspected COVID-19 contamination, but only three (1.6%) were confirmed by RT-PCR. The COVID-19 pandemic resulted in a significant decrease in robotic-assisted surgery. Robotic approach was safe with a low rate of postoperative COVID-19 contamination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus-disease 2019 (COVID-19) first appeared in the Wuhan region of China in December 2019 and has since spread worldwide to become a pandemic resulting in more than 500 000 deaths [1]. In France, complete lockdown was pronounced on March 17th and ended on May 11th 2020. From March 12th, the political authorities announced a so called “plan blanc” and every surgical unit was asked to postpone non-urgent surgical procedures to avoid the saturation of hospitals and intensive care units (ICUs). This decision has delayed surgical management and reduced access to healthcare, particularly for patients with cancer [2, 3]. As a consequence, many scientific societies issued urgent COVID-19 outbreak guidelines to help surgeons prioritise patients [4,5,6].
Robotic-assisted laparoscopic and thoracoscopic surgical procedures have increased over the last decade in various surgical specialties and are now an alternative to open surgery in several indications [7]. This technique involves insufflating gas into the peritoneal cavity. Although no specific data are available on the subject, some people consider that gas insufflation is comparable to aerosol use and could increase the risk of COVID-19 transmission during the procedure, but this remains controversial [8, 9]. During the pandemic, specific recommendations have been issued to reduce this hypothetical risk [10, 11].
SARS-CoV-2, the virus causing COVID-19, is a small virus that can be transmitted via small respiratory water droplets (> 20 μm) and also in aerosolised smaller droplets (< 10 μm) but its transmission by aerosolisation during surgery remains hypothetical. SARS-CoV-2 has been detected in blood and respiratory specimens, but its extra-pulmonary presence and transmission via urine or blood are still unclear [12,13,14].
The aim of this study was to assess the impact of the COVID-19 pandemic on the volume of robotic-assisted laparoscopic and thoracoscopic procedures carried out across 10 academic hospitals in Paris, France and to assess the risk of postoperative COVID-19 contamination.
Methods
Study design
All robotic-assisted surgical procedures carried out in the Assistance-Publique des Hôpitaux de Paris (APHP) during the lockdown period in France were included in this study. The 10 APHP hospitals involved are equipped with 10 surgical Xi robots and 3 Si Intuitive systems© (three academic hospitals have both the Xi and Si system) (Table 1). Robotic systems are currently used in urology, gynaecology, thoracic, paediatric and general surgery.
All patients gave their written informed consent to authorise prospective data collection and retrospective data analysis. The database was declared to the National Board for Informatics and Freedom (Commission Nationale Informatique et Liberté, CNIL, Authorisation #2,218,634) and to the National Institute for Health Data (Institut National des Données de Santé, INDS, Authorisation # MR5214070720).
Data collection
All data from the COVID-19 lockdown period (from March 16th to April 30th 2020) were collected prospectively. A similar period lengthwise (34 weekdays) and a year before was established as a so-called “reference period” to assess the regular volume of activities in daily practice and these data were used for comparison purposes. All patients had a minimum follow-up of 1-month post-surgery.
Data were collected using a shared computerised operative scheduling system common to all academic APHP centres. The following data were collected: age at surgery, sex, body weight, surgical indication, date of procedure and use of the AirSeal© system. COVID-19 screening management, postoperative complications and postoperative COVID-19 status were also recorded for the COVID-19 period.
Surgical management
Every surgical procedure was managed by the local team, and there were not strict recommendations regarding surgical technique during the study. Insufflation for laparoscopic procedures was chosen by the surgeon, AirSeal system was the tri-lumen filtered tube set (ASM-EVAC) and was always used with a device that suctions out the plume. Thoracoscopic procedures did not use insufflation. Post-operative care was standardised in accordance with national recommendations.
COVID-19 perioperative management
Within each academic hospital, COVID-19 screening was carried out before surgery according to crisis board recommendations. For each patient, initial screening consisted of clinical examination for COVID-19 symptoms and of a careful interview. In case of suspicious symptoms, patients were isolated and underwent COVID-19 RT-PCR. According to the RT-PCR results, surgery could be continued, delayed or converted into an open approach. In the late-time period of the study, as RT-PCR were widely available at our institutions, each centre performed systematic COVID-19 RT-PCR on nasopharyngeal swab and some of them a chest CT-scan before surgery.
Patients were hospitalised in a single-room and wore a surgical mask. Oro-tracheal intubation was performed by one anaesthetist wearing FFP2 protection and the surgical staff was asked to remain out of the operating room during intubation. When the patient was COVID-19 suspected, each member of the medical, surgical and anaesthesia teams wore adequate personal protective equipment including goggles, FFP2 masks and protective gowns.
During hospitalisation, any patient with suspected COVID-19 was tested by naso-pharyngeal RT-PCR. To assess the safety of robotic-assisted laparoscopy during the pandemic, contamination was confirmed for every symptomatic patient by postoperative RT-PCR.
Outcomes
Surgical activity, perioperative outcomes and patient demographics were compared between the COVID-19 and the reference period. Postoperative complications were recorded for 30-day post-surgery. Postoperative COVID-19 contamination was considered as a positive RT-PCR within 1 month.
Statistical analysis
Quantitative variables are described as median and inter-quartile range [IQR], and qualitative variables as number and percentage. The two different time periods were analysed first. Pearson’s Chi2 test and the Student’s t test were performed to compare qualitative and quantitative variables, respectively. The COVID-19 period was analysed separately to reveal specific COVID-19 outcomes and to assess robotic surgical procedure safety.
Statistical significance was set at p < 0.05. All tests were 2-sided. Analyses were performed using R version 3.6.2. (2009–2019 RStudio, Inc.) and figures were created using XlStat.
Results
Study population
Overall, 455 robotic procedures were performed during the reference period and 180 between March 16th and April 30th 2020 (COVID-19 period). Mean [IQR] number of cases per system was 40 [31–41] during the reference period and 11 [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] during the COVID-19 period.
Median [IQR] age and weight at surgery were not significantly different between the COVID-19 and reference period (63 years [51–69] vs. 61 years [47–69], respectively; p = 0.6) and (73 kg [62–83] vs. 75 kg [62–85], respectively; p = 0.1) (Table 2). Regarding surgical outcomes, hospital stays were similar (3 days [2,3,4,5,6] during the COVID-19 period vs. 3 days [2,3,4] during the reference period; p = 0.2) and there was no difference in complications between the two groups.
Robotic surgical activity during the pandemic
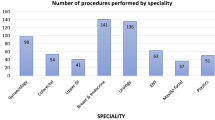
When compared to the reference period, overall activity decreased by 60% and oncological activity by 49% during the pandemic (Table 3 and Fig. 1).
However, the proportion of surgeries performed for oncological reasons was significantly higher during the COVID-19 period compared to the reference period (154/180 (85.6%) procedures vs. 300/455 (65.9%), respectively; p < 0.001) (Fig. 2).
Evolution of oncological and non-oncological procedures during the pandemic. When compared to the reference period, overall activity decreased by 60% and oncological activity by 49% during the pandemic. The proportion of surgeries performed for oncological reasons was significantly higher during the COVID-19 period compared to the reference period [154/180 (85.6%) procedures vs. 300/455 (65.9%), respectively; p < 0.001]
When stratified according to surgical speciality, there were differences in the volume decrease which varied between 41% for paediatric surgery and 81% for gynaecological surgery (Table 3).
Overall, the AirSeal© insufflator system was used for 85 procedures (47.2%) during the COVID-19 period and 174 procedures (38.2%) during the reference period (p = 0.05).
Postoperative COVID status
Of the 180 patients who underwent robotic-assisted laparoscopy during the pandemic, one had already been treated for a COVID-19 1 month before surgery and had a negative RT-PCR test before surgery. Overall, 13 patients (7.2%) presented with suspicious COVID-19 symptoms during their hospital stay post-surgery, but only three (1.6%) of these were confirmed by a positive RT-PCR and the remaining 10 (5.6%) had a negative test (Table 4). Among the positive RT-PCR patients, two underwent lobectomy and the other patient underwent radical nephroureterectomy; regarding the preoperative screening, two patients did not undergo screening, because it had not been set up in the centres at the time and one had a negative chest CT-scan. AirSeal system was used for the patient who underwent radical nephroureterectomy. Finally, one patient with postoperative COVID-19 contamination was managed in the ICU and required oro-tracheal intubation, while the two other patients were managed in a COVID-19 unit without intubation.
All patients in the COVID-19 period underwent a follow-up consultation (face-to-face or teleconsultation) at least 1 month after surgery. The majority (96.1%) had no suspicious symptoms of COVID-19. Seven patients (3.9%) reported minor symptoms that could have been related to COVID-19 (two had fever, two had cough, three had digestive symptoms) but these were not confirmed by RT-PCR and the patients did not need hospitalisation.
Discussion
This multicentre observational cohort study highlights the huge impact of the COVID-19 pandemic on the volume of robotic-assisted surgical procedures carried out in academic centres in Paris, France, with a > 60% decrease in activity compared to a similar reference period. The study also demonstrates that the risk of nosocomial COVID-19 contamination does not seem to be higher with robotic-assisted surgery [15]. The multicentre, prospective design and comprehensive data collection in this study strengthens our results.
Overall, the volume of robotic surgical activity decreased dramatically during the COVID-19 pandemic, but less in oncology compared to other medical specialities. Indeed, the proportion of oncological surgical procedures performed was higher during the pandemic compared to the reference period. This adjustment in activity in oncology was made possible by the speed with which several scientific societies established crisis guidelines based on the stratification of cases to access surgery.
Nevertheless, oncological surgery was affected by a 49% decrease in activity and we are now facing a potential second mortality rebound in oncology populations. Patients treated for cancer have higher COVID-19 related morbidity and mortality than the general population, probably due to immunosuppression induced by systemic treatments [16]. Because of the lockdown, many patients could have neglected their symptoms or failed to consult a physician due to fear, resulting in advanced disease at diagnosis with less treatment options [17]. Although some cancer treatments can be delayed for a few weeks with a low risk of progression, it is not recommended for more aggressive cancers as it can impact outcome and survival [18,19,20]. Moreover, holding off initial therapy could result in the progression of the disease with a more aggressive surgery needed and a higher morbidity. Tumour that initially could be removed, could also grow and become surgically inextirpable because of the delay. For those patients, prognostic can be altered due to the treatment delay. As the diagnosis and treatment of cancers has been delayed by the pandemic globally, the impact on cancer related mortality has to be assessed [21, 22].
At the coming out of the current peak of the pandemic, these key points must be considered to avoid a secondary oncological health crisis. Clinicians are now facing huge challenges: catching up on cancelled operations, maintaining optimal healthcare access while ensuring low nosocomial COVID-19 transmission in hospitals and preventing a second wave of COVID-19.
There is a lack of data regarding virus transmission by aerosolization. For this reason it has been recommended to generate a pneumoperitoneum with a one-way insufflator with an intelligent integrated flow system (AirSeal© system) as a precautionary measure [10]. Associated with this system, it is recommended to use a device that evacuates the plume reducing the loss of gas and a possible virus aerosolization. In our study, we observed a significantly increased use of a one-way insufflator (such as AirSeal©) during the pandemic. The theoretical risk of nosocomial COVID-19 contamination within hospitals at the beginning of the pandemic led to a drastic decrease in surgical activity, especially for robotic procedures in some centres [23]. In addition, several societies recommended laparotomy over laparoscopy because of aerosol generation [24]. To support this hypothesis, previous studies have reported a higher smoke concentration during laparoscopic procedures that could be responsible for the transmission of viruses such as human papilloma or human immunodeficiency virus [25, 26]. Moreover, as SARS-CoV-2 has been found in many bodily fluids (urine, blood, faeces) and could also be located within the peritoneum with a risk of transmission with a pneumoperitoneum [27]. However, as reported by robotic surgical societies such as the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) or the EAU Robotic Urology Section (ERUS), the level of evidence for this mode of transmission is low [10, 11]. These societies advocated the following steps: (i) minimising the use of energy devices; (ii) generating the pneumoperitoneum using a one-way insufflator with an intelligent integrated flow system set up in continuous smoke evacuation and filtration mode; and (iii) COVID-19 testing and risk assessment before surgery.
The results of our study show a very low rate of COVID-19 contamination in the postoperative period. Only three patients tested positive after surgery out of 180 (1.7%). These patients all underwent surgery for oncological indications and the risk of nosocomial transmission was weighed against the high risk of cancer progression. We, therefore, believe that our results strengthen the notion that minimally invasive robotic-assisted surgery is not a risk factor for nosocomial COVID-19 transmission. First of all, the increased use of AirSeal system (ASM-EVAC) associated with a systematic use of adequate protective equipment could have reduced this transmission risk. Moreover, minimally invasive robotic-assisted surgery could actually prevent the risk of contamination by reducing a patient’s exposure during a shorter hospital stay. While patients are at high risk of nosocomial contamination in hospitals that concentrate many COVID-19 patients, robotic surgery is associated with a shorter duration of surgery and shorter length of hospital stay [28,29,30,31].
Among our contaminated patients, only one required admission to an ICU and respiratory support and this patient survived. Conversely, Nepogodiev et al. [32] published a 30-day mortality rate of 24% for COVID-19 positive patients who underwent surgery. However, most patients had non-planned surgeries without undergoing screening and the high mortality rate was partly associated with age > 70 years, American Society of Anaesthesiologists grade ≥ 3, or major surgery. Our careful selection of patients and planned surgeries with screening during the pandemic explain our low morbidity rates.
Compared to other continent, our results regarding the risk of virus transmission during robotic-assisted are consistent with the literature. In Europe, an Italian study evidenced no virus transmission during the lockdown for patients who underwent urological mini-invasive procedures [33]. In America, Moschovas et al. presented 147 patients who underwent robotic assisted radical prostatectomy with no COVID-19 contamination after a 15-day follow-up [34]. Moreover, there is low evidence in the literature about surgical activity modification during the pandemic. In Asia, a multinational study showed a different management of thoracic surgery depending of the considered country. The difference was explained as the result of previous epidemic experience [35].
Our study has several limitations. It could be argued that the preoperative protocol was far from perfect and that even patients without symptoms should have been tested for coronavirus as it is usually performed now. However, at the start of the pandemic, decisions had to be made on procedures in an emergency. Moreover, this study evidences and adds to the literature that, despite a protocol relying on clinical symptoms, robotic-assisted surgery was safe. The retrospective design of our study may have also affected the results.
Conclusion
The volume of robotic-assisted surgical procedures decreased dramatically during the COVID-19 pandemic, although the proportion of oncological robotic surgical procedures increased. We believe the overall decrease in robotic surgical activity was related more to a reassessment of surgical tools and access to ICUs and COVID-19 units rather than a fear of dissemination of the virus with the laparoscopic or thoracoscopic approach. Finally, robotic-assisted laparoscopic and thoracoscopic procedures appeared safe regarding COVID-19 transmission risk in this comparative study.
References
Coronavirus. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 31 May 2020
Pinar U, Anract J, Duquesne I et al (2020) Impact of the COVID-19 pandemic on surgical activity within academic urological departments in Paris. Prog Urol. https://doi.org/10.1016/j.purol.2020.05.001
Campi R, Amparore D, Capitanio U et al (2020) Assessing the burden of nondeferrable major uro-oncologic surgery to guide prioritisation strategies during the COVID-19 pandemic: insights from three Italian high-volume referral centres. Eur Urol. https://doi.org/10.1016/j.eururo.2020.03.054
Ribal MJ, Cornford P, Briganti A et al (2020) European Association of Urology Guidelines Office Rapid Reaction Group: an Organisation-wide Collaborative Effort to Adapt the European Association of Urology Guidelines Recommendations to the Coronavirus Disease 2019 Era. Eur Urol. https://doi.org/10.1016/j.eururo.2020.04.056
Akladios C, Azais H, Ballester M et al (2020) Recommendations for the surgical management of gynecological cancers during the COVID-19 pandemic—FRANCOGYN group for the CNGOF. J Gynecol Obstet Hum Reprod. https://doi.org/10.1016/j.jogoh.2020.101729
Méjean A, Rouprêt M, Rozet F et al (2020) Recommendations CCAFU on the management of cancers of the urogenital system during an epidemic with coronavirus COVID-19. Prog Urol. https://doi.org/10.1016/j.purol.2020.03.009
Fan G, Zhou Z, Zhang H et al (2016) Global scientific production of robotic surgery in medicine: a 20-year survey of research activities. Int J Surg. https://doi.org/10.1016/j.ijsu.2016.04.048
Spinelli A, Pellino G (2020) COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. https://doi.org/10.1002/bjs.11627
Morris SN, Fader AN, Milad MP, Dionisi HJ (2020) Understanding the “scope” of the problem: why laparoscopy is considered safe during the COVID-19 pandemic. J Minim Invasive Gynecol. https://doi.org/10.1016/j.jmig.2020.04.002
(2020) Resources for smoke and gas evacuation during open, laparoscopic, and endoscopic procedures. In: SAGES. https://www.sages.org/resources-smoke-gas-evacuation-during-open-laparoscopic-endoscopic-procedures/. Accessed 4 May 2020
Professionals S-O (2020) EAU robotic urology section (ERUS) guidelines during COVID-19 emergency. In: Uroweb. https://uroweb.org/eau-robotic-urology-section-erus-guidelines-during-covid-19-emergency/. Accessed 7 May 2020
Yeo C, Kaushal S, Yeo D (2020) Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol 5:335–337. https://doi.org/10.1016/S2468-1253(20)30048-0
Chen W, Lan Y, Yuan X et al (2020) Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect 9:469–473. https://doi.org/10.1080/22221751.2020.1732837
Chang L, Yan Y, Wang L (2020) Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. https://doi.org/10.1016/j.tmrv.2020.02.003
Soltany A, Hamouda M, Ghzawi A et al (2020) A scoping review of the impact of COVID-19 pandemic on surgical practice. Ann Med Surg 57:24–36. https://doi.org/10.1016/j.amsu.2020.07.003
Liang W, Guan W, Chen R et al (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21:335–337. https://doi.org/10.1016/S1470-2045(20)30096-6
Gomolin T, Cline A, Handler MZ (2020) The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. https://doi.org/10.1080/09546634.2020.1762844
Wallis CJD, Novara G, Marandino L et al (2020) Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur Urol. https://doi.org/10.1016/j.eururo.2020.04.063
Ginsburg KB, Curtis GL, Timar RE et al (2020) Delayed radical prostatectomy is not associated with adverse oncological outcomes: implications for men experiencing surgical delay due to the COVID-19 pandemic. J Urol. https://doi.org/10.1097/JU.0000000000001089
Russell B, Liedberg F, Khan MS et al (2020) A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Europ Urol Oncol 3:239–249. https://doi.org/10.1016/j.euo.2019.09.008
Hanna TP, Evans GA, Booth CM (2020) Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol 17:268–270. https://doi.org/10.1038/s41571-020-0362-6
Raymond E, Thieblemont C, Alran S, Faivre S (2020) Impact of the COVID-19 outbreak on the management of patients with cancer. Target Oncol 15:249–259. https://doi.org/10.1007/s11523-020-00721-1
Ingels A, Bibas S, Abdessater M et al (2020) Urology surgical activity and COVID-19: risk assessment at the epidemic peak the parisian multicenter experience. BJU Intern. https://doi.org/10.1111/bju.15164
src="/css/img/rcsed_map.png?width=480 TRC of S of E£ TRC of S of E <img, Street height=236" alt="RCSEd E for a larger map" title="RCSEd E for a larger map" /> GDN, Edinburgh, et al Intercollegiate General Surgery Guidance on COVID-19 update. In: The Royal College of Surgeons of Edinburgh. https://www.rcsed.ac.uk/news-public-affairs/news/2020/march/intercollegiate-general-surgery-guidance-on-covid-19-update. Accessed 31 May 2020
Sawchuk WS, Weber PJ, Lowy DR, Dzubow LM (1989) Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol 21:41–49. https://doi.org/10.1016/s0190-9622(89)70146-8
Zheng MH, Boni L, Fingerhut A (2020) Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. https://doi.org/10.1097/SLA.0000000000003924
Peng L, Liu J, Xu W et al (2020) SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. https://doi.org/10.1002/jmv.25936
Ilic D, Evans SM, Allan CA et al (2017) Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009625.pub2
Lanfranco AR, Castellanos AE, Desai JP, Meyers WC (2004) Robotic surgery: a current perspective. Ann Surg 239:14–21. https://doi.org/10.1097/01.sla.0000103020.19595.7d
Shi C, Gao Y, Yang Y et al (2019) Comparison of efficacy of robotic surgery, laparoscopy, and laparotomy in the treatment of ovarian cancer: a meta-analysis. World J Surg Oncol 17:162. https://doi.org/10.1186/s12957-019-1702-9
O’Sullivan KE, Kreaden US, Hebert AE et al (2019) A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 28:526–534. https://doi.org/10.1093/icvts/ivy315
Nepogodiev D, Bhangu A, Glasbey JC et al (2020) Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 396:27–38. https://doi.org/10.1016/S0140-6736(20)31182-X
Motterle G, Dal Moro F, Zanovello N et al (2020) Minimally invasive urologic surgery is safe during COVID-19: experience from two high-volume centers in Italy. J Robot Surg 14:909–911. https://doi.org/10.1007/s11701-020-01099-y
Moschovas MC, Bhat S, Rogers T et al (2020) Managing patients with prostate cancer during COVID-19 pandemic: the experience of a high-volume robotic surgery center. J Endourol. https://doi.org/10.1089/end.2020.0751
Jheon S, Ahmed AD, Fang VW et al (2020) General thoracic surgery services across Asia during the 2020 COVID-19 pandemic. Asian Cardiovasc Thorac Ann 28:243–249. https://doi.org/10.1177/0218492320926886
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
TB: Participated in research design, writing of the paper and performance of the research, UP: Participated in research design, writing of the paper, performance of the research, data analysis and data acquisition, JA: Participated in writing of the paper, performance of the research and data acquisition, JA: Participated in study conception, research design and data acquisition, FA: Participated in study conception, research design and data acquisition, BB: Participated in study conception, research design and data acquisition, De La: Participated in study conception, research design and data acquisition, AEG: Participated in study conception, research design and data acquisition, PA-M: Participated in study conception, research design and data acquisition, PM: Participated in study conception, research design and data acquisition, CP: Participated in study conception, research design and data acquisition, MR: Participated in research design, writing of the paper and performance of the research.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blanc, T., Pinar, U., Anract, J. et al. Impact of the COVID-19 pandemic on oncological and functional robotic-assisted surgical procedures. J Robotic Surg 15, 937–944 (2021). https://doi.org/10.1007/s11701-021-01201-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-021-01201-y