Abstract
The study presents a breakthrough of a balanced charge separation for heterojunction CuWO4-TiO2 cocatalyst to efficiently enhance visible light photocatalytic degradation of ciprofloxacin (CIP). A solvothermal-synthesized nanopyramid-like CuWO4 semiconductor was assembled before sol–gel treatment with TiO2 precursors to generate CuWO4-TiO2 nanocomposites. The optical, structural, and morphological properties of CuWO4-TiO2 were elucidated using UV–Vis DRS, XRD, FTIR, Raman spectroscopy, and TEM/SEM techniques. The UV–Vis DRS spectroscopy of as-synthesized CuWO4-TiO2 cocatalyst demonstrated enhanced visible light absorbance. The XRD patterns of CuWO4-TiO2 revealed a triclinic phase nanocrystal. The O-Ti–O functionality was confirmed by FTIR spectroscopy. The photoactive bands corresponding to anatase redshift were observed from Raman spectroscopy of CuWO4-TiO2 nanocomposite. The PL studies attributed this redshift to the elevated extra energy bands that aid electron/hole pair charge separation in a co-catalyst heterojunction CuWO4-TiO2 nanocomposite afforded by embedding CuWO4-MOF within TiO2 crystalline. The TEM showed that un-sintered CuWO4.MOF mimicked a pyramidal shape and converted to nanoflakes upon sintering, while TiO2 and CuWO4-TiO2 retained a tetragonal shape. The photocatalytic activity of CuWO4-TiO2 cocatalyst was studied using CIP, as a model pollutant. The innovative design of 5CuWO4-TiO2 charge separation nanocomposite completely degraded 10 mg L−1 CIP solution at pH = 6.31 (natural pH) and 9 under 120 min of sunlight irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) photoactivity was discovered in 1972 by Fujishima and Honda during their work on photoelectrochemical water splitting (Barde et al. 2022; Honda 1972). The photo-functionality of TiO2 made it a research benchmark for photocatalytic advanced oxidative reactions (AOR) (Xue et al. 2021). This photoactivity is due to the TiO2 light absorbing O2− → Ti4+ band with an energy bandgap (Eg) of 3.0–3.21 eV (Kaur et al. 2021; Bharagav et al. 2022). The dominance of ultraviolet (UV) active O2− → Ti4+ large Eg favors the rapid charge recombination rate of photo-excitons and opposes the much-required net chemical reaction of TiO2 and O2. High recombination limits the generation of free radicals responsible for initiating the degradation of pharmaceuticals (Luo et al. 2016). The UV-restricted TiO2 photoactivity prompted a search for other oxygen evolution photocatalysts (OEP) with a visible light response. Copper tungstate (CuWO4) emerged as a favorable OEP due to visible light indirect narrow Eg of 2.2–2.4 eV (Raizada et al. 2020). A narrow bandgap makes CuWO4 suffer from electron (e−)/(h+) hole pair separation. The TiO2 rapid charge recombination and CuWO4 low charge separation are limitations at opposite ends. This gave rise to a global curiosity aimed at designing viable charge management nanotechnology by pairing TiO2 and CuWO4.
Central to the charge balance management quest is the fabrication protocols to afford a stable visible light-responsive CuWO4-TiO2 (CWT) (Bharagav et al. 2022). This is premised on three realities—(1) CuWO4 bandgap is intrinsically dependent on the structural framework and coupling of octahedral CuO6 and WO6 (Raizada et al. 2020; Hu et al. 2019). This reality infers that the material design sequence also controls the redshift from TiO2 UV-active Eg = 3.21 eV to a visible light-driven CWT photocatalyst. Secondly, CuWO4 crystals are susceptible to photo-corrosion distortion when exposed to alkaline photo-oxidation reactions (Raizada et al. 2020). Thirdly, TiO2 has a tunable Eg and a high photostability (Xue et al. 2021; Mmelesi et al. 2023). The latter realities imply that embedding CuWO4 into TiO2 is an architectural viability for a photostable visible light active CWT (Bharagav et al. 2022).
CWT nanomaterials were previously prepared by coprecipitation, microwave-assisted, alcoholysis sol–gel-assisted, hydrothermal, and solvothermal techniques (Liu et al. 2019). Some found that TiO2 transfers electrons into the solid CuWO4 structure than Cu2+ ion (Luo et al. 2016). Contrary findings argued that the narrow Eg of 2.22 eV for CuWO4 was a motive force behind the excitation of valence band (VB) electrons and their subsequent photoexcitation transfer through Cu2+ channels onto TiO2 lattice (Kaur et al. 2021). These varying photocatalytic mechanisms are a testament that the photocatalytic strengths of CWT are controlled by synthesis protocols, structural geometry, and catalyst loading (Bharagav et al. 2022). A recent study proved that a Cu-MOF (copper metal–organic framework) gives photocatalysts a highly defined structural geometry, particle sizes, and porous crystallinity that stimulates light penetration (Xue et al. 2021).
Therefore, the current study aims to authenticate that an architectural nanotechnology marriage between CuWO4-MOF and TiO2 can yield CuWO4-TiO2 (CWT) cocatalyst that allows visible light penetration for sufficient facilitation of e−/h+ pair charge separation. The photocatalytic oxidative performance of CWT was evaluated using ciprofloxacin (CIP) as a model recalcitrant pharmaceutical contaminant of emerging concern (CEC).
Experimental section
Materials and reagents
The reagents used for material synthesis were titanium isopropoxide (TTIP) (97%), 2-propanol (99.5%), ammonium hydroxide solution (25%), trimesic acid (1, 3, 5-benzene tricarboxylic acid, H3BTC) (95%), copper nitrate—Cu(NO3)2.3H2O (99%), tungsten oxide (> 99%), ethanol (96%) and dimethyl sulfoxide (DMSO) (98%). Hexacyanoferrate(III) ([Fe(CN)6]3−/4−) was used as an electrolyte for electrochemical analysis of EIS and CV. Ciprofloxacin (> 99%), NaOH (99%), and HCl (36%) were used for photocatalytic evaluation solutions. All reagents and chemicals were of analytical grade and used as acquired from Merck Group, South Africa. The deionized water (H2O) used for all experiments was provided by the Millipore-Q water system.
Synthesis of nanoparticles and the nanocomposites
TiO2 nanoparticles
Sol–gel method was adopted for TiO2 nanoparticle synthesis (Wu and Chen 2004; MacWan et al. 2011; Restrepo et al. 2010). A solution of 10 ml of TTIP was dissolved into a magnetically stirred 20 ml of 2-propanol. Then, 50 ml of deionized water was added to the solution. The mixture was then activated dropwise by adding 6 ml of NH4OH. The mixture was allowed to age for 0.5 h before raising the temperature to 60 °C for 1 h. The temperature was then lowered to room temperature for 2 h with continuous stirring. The contents were cooled and centrifuged at 900 rpm for a third of an hour. The supernatant and filtrates were left in contact overnight to allow further intercalation of N-atoms from NH4OH onto TiO2 surfaces. The resulting mixture was re-centrifuged after 16 h, and the supernatant was discarded. The white solids were sequentially cleaned with deionized water and ethanol. The cleaning was repeated twice before drying at 90 °C overnight. The white TiO2 crystals with a faint yellow tinge were calcined at 400 °C for 2 h.
CuWO4-MOF nanocomposites
Equimolar solutions of 4 mmol were prepared by dissolving Cu(NO3)2.3H2O and WO3 in separate ethanol solutions and sonicated for 0.5 h. The two solutions were then combined and sonicated for a further 0.5 h. CuWO4-MOF nanopyramids were prepared using a previous solvothermal procedure with significant modifications (Liu et al. 2019; Wu and Chen 2004; MacWan et al. 2011). A 1.0 g (0.00476 mol) of H3BTC was dissolved in a solvent solution mixture made of 20 ml DMSO, 20 ml of 2-propanol, and 20 ml of deionized water (v/v 1; 1; 1). Then, the sonicated tungstate copper oxide solution (CuWO4) was added to the resulting H3BTC mixture. The mixture was further homogenized for 0.5 h at room temperature before being transferred into a 250-ml thermal autoclave and heated at 160 °C for 16 h. The resultant blueish crystals were centrifuged and washed twice with deionized water and ethanol. The solids were dried at 90 °C for 24 h before sintering at 400 °C for 2 h.
CuWO4-TiO2 (CWT) nanocomposites
A series of xCuWO4-TiO2 (CWT) samples were prepared (Bai et al. 2022; Xiong et al. 2015), where x refers to the mass of 0, 5, 100, and 500 mg added as the as-prepared CuWO4-MOF nanocomposite. A desired mass of as-prepared CuWO4-MOF was sonicated into 20 ml of ethanol. In a separate beaker, 10 ml of TTIP was dissolved into 20 ml of 2-propanol. Then, 50 ml of deionized water was added under constant magnetic stirring. The solution was activated dropwise by adding 4 ml of NH4OH and agitated further for 0.5 h. The resulting titania solution was dropwise transferred into the CuWO4 solution while stirring. The resultant mixture was agitated for 0.5 h. The blueish-white mixture was further turned basic by a dropwise addition of 2 ml of NH4OH. The solution was then evaporated at 60 °C for 1 h before cooling to room temperature and centrifuging at 900 rpm for 0.5 h. Both the precipitates and supernatant were left in contact for 16 h to allow nitrogen/titania intercalations which leads to oxygen deficiencies. The mixture was then centrifuged again the next day, and the precipitate was collected. All the resulting CuWO4-TiO2 (CWT) nanocomposites were triple-cleaned using the centrifugation method by alternating distilled water and ethanol as cleaning solvents. The white solids were dried at 90 °C for 24 h before sintering at 400 °C for 2.5 h.
Materials characterization
The absorbance of nanosolids was analyzed directly without any further sample treatment using ultraviolet–visible diffuse reflectance spectroscopy (UV–vis DRS) measured between 200 nm and 800 nm range by PerkinElmer UV/Vis/NIR spectrometer Lambda 1050. Horiba Jobin Yvo Fluorolog-3 was used to acquire the photoluminescence (PL) spectra for the nanosolids dissolved in ethanol. The charged nature was measured from cyclic voltammetry (CV) and impedance (EIS) using Biologic EC-Lab. The nanosolids were first dissolved in deionized water before dipping the cleaned electrodes into each of the prepared paste-like sample solutions. The surface micromorphological nature and elemental composition of samples coated with carbon tape were visualized and recorded using the SEM-JOEL JSM-IT300 series equipped with EDS (Oxford X-MAXN). Microstructures and shapes were resolved using TEM-Tecnai G2F2O X-Twin MAT (Eindhoven, Netherlands) operating at an accelerating voltage of 100 kV. Functional groups of each nanosolid were recorded from 32 scans acquired from the PerkinElmer FTIR spectrometer coupled with Frontier-Spectrum 100 spectrometer as Fourier transform-infrared (FTIR) spectra of nanosolid and KBr pellet mixtures converted into a disk. The solid samples were assessed for X-ray diffraction (XRD) without any further sample treatment by exposing each sample to a laser from a Rigaku SmartLab X-ray diffractometer with Cu Kα radiation (λmax = 0.154059 nm) operated at 40 kV. Raman spectra of untreated solid samples were acquired using Witec Raman spectrometer Alpha 300, TS 150 (Germany), coupled with an operating laser power source of 532 nm.
Electrode preparation
A glassy carbon electrode (GCE) was modified following the procedure adopted by previous study (Nepfumbada et al. 2024). The GCE working electrode was sequentially cleaned by applying alumina slurry solutions of concentrations 1.0, 0.3 0.05 μm and polishing with polishing pads. The working electrode was then dipped into a mixture of 1:1 v/v of water/ethanol under sonication. In a separate experiment, 5 mg was taken from each of the samples TiO2, CuWO4, and CWT and tranerred into independent sample vials containing 5 ml of deionized water. The resulting solutions were independently sonicated for an hour. Exactly 8 μL of each of the three solutions was independently drop casted on separate clean GCE working electrodes. The electrodes were air dried for 16 h. Biologic potentiostatic mode technique was used to analyze voltammetry (CV) and electrochemical impedance spectroscopy (EIS) with the aid of instrument AUD83909 (Autolab, South Africa). The electrolyte used for all analysis was hexacyanoferrate(III).
Photocatalytic degradation evaluations
CuWO4-TiO2 (CWT) nanocomposite was examined for photocatalytic degradation of ciprofloxacin (CIP) under visible light and then natural sunlight. Four separate 10 mg L−1 of CIP solutions of 120 ml each at pH = 3, 5, 7, or 9 adjusted using 0.1 mol L−1 of HCl and 0.1 mol L−1 of NaOH while stirring were prepared. An aliquot of 2.5 ml was sampled from each of the four solutions. A 50 mg of CuWO4-TiO2 nanoflakes was dispersed in each of the solutions. The solutions were equilibrated in the dark while stirring for 20 min. Another 2.5 ml aliquot was sampled from each of the solutions. The solutions were transferred into a 1000-ml photoreactor equipped with 250 watts of visible light, a three-neck jacketed reactor, an operating magnetic stirrer, and a circulating water chiller system. An aliquot of 2.5 ml of CIP solution was sampled for each 20 min for the 120 min of the reaction under visible light lamb operation. The sampled aliquots were passed through a 0.1 µm Simplepure™ syringe filter. The PerkinElmer UV/Vis/NIR spectrometer Lambda 1050 hosting two cuvette spots was utilized to monitor the absorbance spectra of CIP residual concentration. One cuvette served as background and the other as a sample holder for an analyte. The photodegradation rate was computed from Eq. 1 (Pei et al. 2021; Bibi et al. 2021).
where [CIP]0 and [CIP]t are CIP initial concentration and CIP concentration at time (t). The kinetic model of best fit was also evaluated from Eq. 2 (Bibi et al. 2021; Bahramian et al. 2023; Sarafraz et al. 2020) and 3.
where Kapp denotes the rate constant at time (t) during photocatalytic irradiation (Bahramian et al. 2023).
Results and discussion
The nanocomposites prepared from the titanium and tungsten materials are explored in terms of their fabrication, optimization and use for the photocatalytic degradation of ciprofloxacin. Various parameters were investigated, and their characterization and observations discussed based on the techniques used to explore their properties.
Optoelectrical properties
In Figure 1a, UV–Vis DRS for CWT samples showed an apparent Urbach tail that increased with increasing CuWO4-MOF dosages. This redshift suggests that CuWO4-MOF introduced elevated electronic states above the valence band of TiO2 forming 5CWT, 100CWT, and 500CWT samples (Tan et al. 2019). Figure 1b shows Tauc plots and Eg for samples as calculated from De Broglie wavelength relationship—Eq. 4 (Yuguru 2022; Meenakshi et al. 2023).
where the parameters \(\lambda , h\), and n represented the junction wavelength, Planck constant, and some linear constant factor for bandgap energy changes. The reduced Eg for TiO2 from the reported UV active 3.21 eV to 2.99 eV symbolizes the effect of NH4OH nitriding (Pei et al. 2021). The measured Eg of 2.32 eV for CuWO4 agrees with literature recorded characteristic Eg range of 2.22 eV to 2.4 eV (Raizada et al. 2020; Hu et al. 2019; Meenakshi et al. 2023). The Tauc plots further showed that increasing CuWO4-MOF dosages inside CWT samples decreased the bandgap trend as—TiO2 (2.99 eV) > 5CWT (2.90 eV) > 100CWT (2.78 eV) > 500CWT (2.65 eV) > CuWO4 (2.32 eV). The trend shows that the Eg of CWT samples is sandwiched between TiO2 and CuWO4 bandgaps despite the observable Urbach tail redshift for CWT samples. This suggests that the elevated electronic state introduced by CuWO4-MOF is responsible for e−/h+ pair charge recombination and separation management (Tan et al. 2019). Photoluminescent spectra of photogenerated e−/h+ charge pair excited at 353 nm for TiO2 and 302 nm for CuWO4 and CuWO4-TiO2 samples are shown in Fig. 1c. The largest intensity reflects that TiO2 reflects the highest e−/h+ pair recombination. The decreased intensity for CWT implies a reduced e−/h+ charge pair recombination in comparison with TiO2. This ties in with the suggestion that CuWO4 introduced extra energy bandgap bridges causing Urbach redshift toward visible light edges under UV-DRS analysis. These heterogenous bridges aid the separation of e−/h+ charge pair by accepting and linearly migrating electrons away from the positively charged holes of O-Ti–O excited valence band atoms across to conduction bands of CWT through an electric field.
A pH drift method was employed to evaluate the point of zero charge (pHPZC) of samples between pH = 3 and pH = 11, Fig. 1d samples (Bibi et al. 2021). The pHpzc was determined as; TiO2 = 3.2, CuWO4 = 4.8, 5CWT = 3, 100CWT = 4.15 and 500CWT = 4.2. The pHpzc = 3.2 for TiO2 deviated from the literature-reported (Pei et al. 2021) pHpzc = 6.5. The decrease in pHpzc for TiO2 reflects the dominance of electronegative N-atoms introduced by NH4OH dropwise nitriding. The pHpzc for TiO2-containing samples convergence toward a zero as pH = 6.5. This suggests that the literature reported pHpzc = 6.5, which may also be TiO2 isoelectric point (IEP). The increase to pHpzc = 4.15 for 100CWT and pHpzc = 4.2 for 5 00 CWT shows that increasing CuWO4 dosages affects the nature of CWT electrical charge. Figure 1e shows interfacial charge transfer resistance for samples TiO2, CuWO4-MOF, and 500CWT acquired from electrochemical impedance spectroscopy (EIS) using EIS Nyquist plots. The arc radius of the samples in the EIS curves increased in the order of TiO2 > 500CWT > CuWO4-MOF. Since a bigger arc radius for TiO2 implies a larger charge transfer resistance, it is prudent to suggest that charge migration increases in the order of CuWO4-MOF > 500CWT > TiO2 (Vinesh et al. 2022). The smaller radius of CuWO4 implied that it has a high propensity to enhance interfacial charge migration for 500CWT (CuWO4-TiO2). The superior charge migration shown by CuWO4-MOF cannot singularly enhance photocatalytic reactions. CuWO4-MOF photocatalysts also needs to be complimented by a suitable conduction band (CB) position with minimum electrochemical potentials of − 0,41 V for H/H+ conversion, − 0.33 V for O2/O2− conversion, -0.24 V for CO2/CO2− conversion, and 2.73 V for OH,H+/H2O conversion (Marschall 2014). The cyclic voltammetry curves in Fig. 1f(Inset) showed that CuWO4-MOF has a pronounced CV curve in comparison with seemingly flat curves for TiO2, 5CWT and 500CWT. Further analysis (Fig. 1f main curves) revealed that the flat curves are also cyclic with increased edges for 5CWT and 500CWT compared to TiO2 sample TiO2. These findings support the EIS suggestion of a highly conducting CuWO4-MOF in 500CWT samples promoted electron flow. By extension, this means CuWO4-MOF enhances separation of e−/h+ in the TiO2 lattice of CWT nanocomposite.
Functional and crystallographic properties
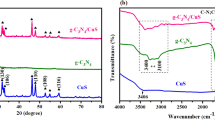
Figure 2a provides FT-IR spectra for synthesized pale-yellow TiO2, pale-yellow CuWO4-TiO2 (CWT), black CuWO4 calcined at 400 °C and uncalcined yellowish green CuWO4. TiO2 anatase characteristic peaks were confirmed at 469 cm−1 as Ti–O, 794 as Ti–O-Ti linkages, and 1623 cm−1 as Ti–OH vibrations. The wavenumber difference of 70 cm−1 calculated between asymmetric peaks of CuWO4 sample at 1582 cm−1 and 1652 cm−1 is below 200 cm−1. This indicated that trimeric acid organic ligand has two coordination modes bridging two dentates (Ramezanalizadeh and Manteghi 2016). This suggests that CuWO4 is a metallic organic framework complex with different energy levels. The probable two dentate interaction was evidenced at 1114 cm−1 as a Cun+-O-Wn+ band for both sintered and non-sintered CuWO4. Previous work also identified this Cun+-O-Wn+ band around 800 cm−1 to 900 cm−1 (Anucha et al. 2021). For CWT samples, the Ti–O band broadens and shifts to environments below 800 cm−1. This observation indicates a possible co-existence of Ti–O bonding interaction, organic ligands known to absorb at 730 cm−1, deformed W–O band of tetrahedral WO4 peaks at 486 and 945–1 and stretching bands of Cu–O peaks at 614 and 728 cm−1. Other researchers identified the Cu–O bond at 745 cm−1 and the W–O bond at 910 cm−1 (Sarwar et al. 2023). The emerging bond at around 2066 cm−1 for 100CWT may be linked to W = O or W–OH bond anchored onto a surface of metal oxide. A recent study made a revelation that oligomerization effect of WO3 may lead to an introduction of a new FTIR vibrations around 2000–2100 cm−1 region when reacted with metal oxides (Jaegers et al. 2019). A previous study showed that this tungsten (W) atoms bands appear around this region but if bonded with atoms such as sulfur it appears as W-S band at higher wavenumbers range of around 2100 cm−1 (Haghighi et al. 2021). The reduced stretching of the Ti–OH band for CWT samples is suggestive of a different structural environment. This may also be due to O-W–O band capable of titrating surface acidic and basic Ti–OH in the region 2200–2900 and 3400 to 3700 (Jaegers et al. 2019). This led to a flattened FTIR spectra in this region leaving a significant appearance of Ti–OH and W-OH existing around broad peak region of 2066 cm−1.
Figure 2b shows XRD phase properties for TiO2, CuWO4, and xCuWO4-TiO2 (CWT) indexed to 8.8 nm tetragonal planar anatase (JCPDS No. 00–064-0863) (Nagakawa and Nagata 2021), 9.1 nm triclinic phase (ICSD no. 16009), and 8.3 nm tetragonal phase, respectively. The CuWO4-TiO2 samples with CuWO4 dosages below 100 mg showed an insignificant shift of 0.01°. This implies that these samples retained the desired photoactive anatase patterns of TiO2. There is an emerging peak at 22.80° along 110 planes for 100CWT and 500CWT. The emergence of this peak at lower values than the intense peak at 25.31° characteristic of TiO2 anatase suggests that CuWO4 was embedded inside the TiO2 crystal lattice. This arrangement suggests that CuWO4 has been architecturally embedded within TiO2 as per the desired design to avoid photo-corrosion against oxidative reactions even under a basic medium. The emerging peak is also consistent with PL and UV–Vis DRS suggestion that CuWO4 introduces electronic bands within CWT. The reduction of tetragonal phase crystal interplanar size from 8.8 nm to 8.3 nm indicated that 0.074 nm Ti4+ ionic radius was substituted by 0.087 nm Cu2+. ionic radius. This substitution reduces size by allowing O-Ti–O to donate electrons to a larger Cu-atom capable of inwardly mobilizing subatomic particles better than TiO2. charge difference between Ti4+ and Cu2+ triggers the creation of oxygen vacancy for maintaining charge neutrality through Jahn–Teller effects (Bahramian et al. 2023).
Raman spectra displayed in Fig. 2c–d represent the phase purity of TiO2, CuWO4, and CuWO4-TiO2. TiO2 samples in Fig. 3c showed the Raman modes: Eg1 (at 146.9 cm−1), B1g (at 399.99 cm−1), A1g (at 519 cm−1) and Eg (at 640.12 cm−1). These TiO2 bands were previously identified at lower wavenumbers of 141, 390, 512, and 634 cm−1 (Luo et al. 2016). The redshift to a higher wavelength in the current work is due to nitriding. The Eg1 band identified at 146.9 cm−1 symbolizes a photoactive TiO2 anatase (Bahramian et al. 2023). For the CuWO4 sample, the peaks at 275 cm−1 and 328 cm−1 confirmed the triclinic phase of the bending δ(O–W–O) identified at FTIR wavenumber of 146.9 cm−1 (Loka et al. 2023). For the CWT sample, the emerged peaks at 88.99 cm−1 and 201.61 cm−1 confirmed the interactions between CuWO4 and TiO2. As per De Broglie’s law from Eq. 1, Moloto and coworkers ascribed Eg1 band redshift from higher energy frequency (v) wavelength (UV) to lower frequency wavelength (visible light) to electron migration from O-Ti–O donor bond categorized by reduced band strength (Moloto et al. 2021).
Morphological surface properties
Morphological investigations of TiO2, CuWO4, and CuWO4-TiO2 are given from SEM in Fig. 3ai–aiii and TEM in Fig. 3bi–biii. Figure 3bi shows agglomerated cubic-shaped structures consistent with a tetragonal phase revealed from XRD phase analysis. The SEM images from Fig. 3ai and aii revealed that CuWO4-MOFs formed a pyramid-like structure. In our research institute, a previous study by Abera et al. (Ambaye et al. 2022) showed that a pyramid-like shape emanates from solvothermal synthesis using Cu precursors and trimeric acid which forms ultimately octahedral Cu-MOF with a flake-like/paddle-wheel-like geometry. The current study reveals that the flake-like/paddles represent a collapse of nano-pyramidal Cu-MOF at elevated temperatures as indicated in Fig. 3bii TEM after sintering CuWO4 at 400 °C for 2 h. The pyramid-like shape concurs with XRD analysis that CuWO4 is a triclinic phase. XRD Gaussian model correlation to the triclinic phase suggests that even the stacked flower-like structures from Fig. 3ai inset represent a triclinic phase in a bipyramid crystal. This is further supported by previous revelations (Feng et al. 2017) that hydrothermal synthesis of MOF performed below 18 h usually produces a mixture of pyramid-like particles and other shapes such as nanotubes. Hence, CuWO4-MOF crystals earned the name CuWO4 nanopyramids in this current work. TEM micrographs of CuWO4-TiO2 composite in Fig. 3biii taken at a 20 nm and 10 nm showered TiO2 nanocomposite image mimicry of a shape between a distorted CuWO4 nanopyramid and a distorted TiO2 nanoparticle tetragonal planar. These CuWO4-TiO2 mix shapes in Fig. 3biii affirm TiO2 and CuWO4 interactions to form Ti–O-Cu–O-W–O- arrangement. Therefore, CWT is bonded as TiO2-CuWO4.
Photocatalytic evaluation of CIP degradation using CWT
Photoactive CuWO4-TiO2 (CWT) nanocomposites were chosen for photocatalytic performance evaluations due to the observed superior charge separation, improved redshift, and light penetrable anatase phased nanoflake geometry as described under the results and discussion section for characterization. Preliminary studies on photocatalytic potential for ciprofloxacin (CIP) were done using TiO2, and a series of xCWT, namely: 5CWT, 100CWT and 500CWT (where CWT refers to CuWO4-TiO2 and the prefix x represent either 5 mg, 100 mg, and 500 mg of CuWO4 contained in CWT. As shown in Fig. 4a–b, TiO2 photodegraded just over 70% of CIP, whereas CuWO4-MOF did not show any significant photocatalytic efficiency for CIP despite the corresponding EIS spectra having displayed better electron migration and PL spectra showing reduced recombination. This suggests that the enhanced electron migration of CuWO4 has conduction bands (CB) minima with electrochemical potentials below − 0,41 V which limits CuWO4 from converting H into H+ and also unable to convert O2 to O2•− as this reaction also needs a threshold of − 0.33 V (Marschall 2014). This means CuWO4 sample is unable to produce necessary reactions to separate e−/h+ pair charge and to sufficiently generate free radicals that can propel photocatalysis. A 5CWT design was used for optimization because it showed the highest photocatalytic efficiency for CIP.
Effect of pH on photocatalytic degradation of CIP using CWT
Figure 4c–f displays the photocatalytic performance of 50 mg of 5CWT dissolved into 120 ml solutions of 10 mg L−1 CIP monitored at pH = 3, 5, 7, and 9. The dark experiments adsorption conducted for 20 min at pH = 3, 5, 7, and 9 removed 4%, 21%, 9.9%, and 17% of CIP in comparison with overall photocatalytic removal efficiency of 35%, 60%, 44%, and 74%, respectively. The variations in adsorption efficiencies suggest that CIP and 5CWT surface interactions are pH-dependent. The low removals at pH = 3 may be due to high attraction between –OH radicals and protons (Bibi et al. 2021), and/or repulsion between h+ and positively charged CIP species existing at pH < 5.5 (Sarafraz et al. 2020; Ngo et al. 2023). The moderate removal of 60% at pH = 7 is linked to the zwitterionic form as CIP is amphoteric (Ngo et al. 2023). The highest efficiency observed at pH = 9 suggests that the addition of –OH increased CIP speciation density and led CIP to precipitate near active sites present on the surfaces of 5CWT.
Visible light illumination photocatalytic performance of CWT with varying CuWO4 dosages
Figure 4a and f provides UV–Vis DRS that showed that 10 mg L−1 of CIP solution at pH = 9 was photodegraded to 80% by TiO2, and 86% by 5CuWO4-TiO2 nanocomposite under visible light. TiO2 nanoparticles photodegraded 80% of CIP under visible light because of redshift caused by the nitriding effect. The high removal efficiency of 86% shown by 5CWT confirms the propensity of CuWO4-MOF to enhance photocatalytic charge migration by lowering electron/hole recombination and increasing charge as per PL results (Bahramian et al. 2023). The simultaneous decrease of ciprofloxacin peaks at 271 nm and 225 nm further suggests that photodegradation of CIP at pH 9 proceeds by at least more than one pathway.
The role of scavengers
The role of 5CWT reactive oxidative species was established by quenching superoxide (O2●−), hydroxyl radicals (●OH), and holes (h+) using p-benzoquinone, 2-propanol, and EDTA, respectively. As shown in Fig. 5f, the addition of p-benzoquinone completely haltered CIP degradation. This implied that O2●− was the primary initiator for CIP photodegradation. This suggests an effective e−/h+ charge pair separation that isolated and allowed O2●− to catalyze the reaction. The unchanged efficiency after adding 2-propanol implied that ●OH radicals had no significant role. This may be due to the repulsion between ●OH radicals and negatively charged CIP speciation at pH = 9. The trapping of h+ by EDTA reduced the removal efficiency from 86 to 44%. This suggests that h+ are involved in at least a single reaction mechanism pathway after degradation is initiated by O2●−. A photodegradation study for carbamazepine showed that h+ species dominated when CuWO4 was used (Anucha et al. 2021).
Effect of natural sunlight irradiation
Figure 5a and b provides spectra for 120 ml of 10 mg L−1 CIP solution degraded by 50 mg of 5CuWO4-TiO2 (5CWT) at pH = 9 and 6.3 (natural pH of dissolved CIP) under natural sunlight, respectively. The results showed that sunlight aided a ~ 100% photodegradation efficiency of 10 mg L−1 CIP at both solutions pHs within 120 min. This suggests that the 4% UV light availed by natural sunlight directly excited the UV active Ti \(\to\) O2 band of 5CuWO4-TiO2 to generate additional oxidative species. This implies that CuWO4 aids 5CWT to adsorb 46% visible light flux of sunlight while the 4% UV light portion directly stimulates more electron migration from the TiO2 valence band to the conduction band.
Kinetics of CIP photodegradation by CuWO4-TiO2 under sunlight
Kinetic probing experiments for 5CuWO4-TiO2 were conducted over 30 min by illuminating 120 ml of 10 mg L−1 CIP at pH 9 using natural sunlight as shown in Fig. 5c. The photocatalytic degradation half-life (t1/2) was achieved in 5 min. At least 69% of 10 mg L−1 CIP was converted by the end of a 30-min kinetics experiment. The fitted data showed that the mechanism preferred the pseudo-second-order model (r2 = 0.91) over the pseudo-first-order model (r2 = 0.54). This implied that (i) the reaction rate constant depends (Kapp) on two factors and (ii) the rate constant for half-life reaction depends on CIP concentration. This suggests that the rate-determining step depends on the solution pH and CIP concentration. Equation revealed that kapp = 0.116 mol.min−1.
Plausible photocatalytic degradation mechanism
The revelation from XRD spectra that 5CWT has an increased crystallinity mimicry of TiO2 tetragonal anatase, the observed reduced PL intensity of 5CWT in comparison with TiO2 and red-shift bands from UV–Vis DRS (and Raman) spectra is a possible indication for a reduced charge carrier recombination, increased e−/h+ charge pair separation and enhanced visible light photoactivity of 5CWT, respectively. These possibilities prompted the calculation of valence band (VB) position from Eq. 4 and conduction band (CB) positions from Eq. 5 using Mulliken electronegativity theory (Feng et al. 2017) as a strategy to establish charge pair separation and transfer mechanism involved in photodegradation of CIP by CuWO4-TiO2 (Bharagav et al. 2022).
where EVB and ECB, χ, Eg, and Ee represent VB and CB positions, electronegativity of the constituent atoms, bandgap, and energy of free electrons on the hydrogen scale ca. 4.5 eV, respectively. The TiO2 VB position was 2.805 eV and the CB position was–0.185 Ev while the CuWO4 VB position was 2.89 eV and the CB position was 0.55 eV vs NHE. Figure 6e. Both the CB and VB of TiO2 semiconductor electrochemical potential rest above their corresponding CB and VB of CuWO4 (Marschall 2014). This staggered energy level condition favors type-II heterojunction and Z-scheme. A possibility for type-I heterojunctions is eliminated because it requires that both the VB and CB of one semiconductor be sandwiched in-between the VB and CB energy of the other semiconductor (Annamalai et al. 2023). A type-III is also improbable because it requires that both the VB and CB of either TiO2 or CuWO4-MOF rests above both VB and CB of the other semiconductor (Marschall 2014). For type-II heterojunction, the electrons should migrate from a higher 0.395 eV vs RHE = TiO2 CB position to a lower 0.55 eV vs RHE = CuWO4 CB. The positively charged CuWO4-MOF CB position of + 0.55 eV vs NHE reveals that CuWO4-MOF serves as oxygen evolution photocatalyst (OEP) that scavengers the electrons necessary to reduce oxygen to superoxide anions (O2●−) (Bharagav et al. 2022; Anucha et al. 2021). If this TiO2 CB electrons migrate to occupy CuWO4-MOF CB, the new CB energy of 0.55 eV vs RHE will incapacitate these electrons from converting O2 to O2●− (O2/O2 → -0.33 eV vs RHE). This means the TiO2 CB electrons with − 0.395 eV vs RHE are responsible for the generation of O2●− superoxide scavenged by p-benzoquinone. This suggests that as sunlight irradiates 5CWT nanocomposite, the presence of CuWO4-MOF with smaller Eg of 2.32 eV creates energy centers between the VB and CB of TiO2 (Kaur et al. 2021). These electrons trapping energy centers cause absorbance edge redshift enabling 5CWT to absorb visible light. The Cu2+/Cu+ and W6+/W5+ redox half-reactions enhance the electron trapping by the electronic energy centers (Bai et al. 2022). This process reduces recombination of electrons and holes from TiO2 VB by promoting migration from TiO2 VB to TiO2 CB (Anucha et al. 2021). The visible light irradiation led to a movement of negatively charged excited electrons occupying CuWO4-MOF CB to recombine with isolated positively charged holes from TiO2 VB. This has two effects: Firstly, it suppresses the recombination of TiO2 VB holes and CB electrons; and secondly, this continuous opposite migration of charge carriers along the heterojunction of TiO2 and CuWO4 gives rise to localized electric field that help to transfer these charge carriers across heterojunction to avoid recombination. The inter-migration of electrons between CuWO4 CB and TiO2 VB suggests a Z-scheme heterojunction. Since CUWO4-MOF serves as OEP, then TiO2 serves as a hydrogen evolution photocatalyst (HEP) (Bharagav et al. 2022). That means the holes (h+) migrate from CUWO4-MOF VB to accumulate onto the interface of TiO2 VB. The interparticle transfer of holes is simplified by the comparable closeness of the CuWO4-MOF VB position of 2.89 eV and TiO2 VB position of 2.805 eV. This closeness of CuWO4 VB and TiO2 VB in which the h+ migration occurs has been reported to increase occurrence of a heterogenous Z-scheme-like mechanism (Bao et al. 2021). Since the h+ from TiO2 VB and e− from CuWO4-MOF recombine in the TiO2 VB, the positively charged h+ from CuWO4-MOF remains isolated to isolated and accumulate on the interface of TiO2 VB without occupying TiO2 CB. The electron potential 2.805 eV vs RHE enables these isolated h+ charge carriers from CuWO4-MOF VB to either directly react with CIP through oxidation or react with H2O to form –OH hydroxyl radicals (H2O/–OH = 2.8 eV vs RHE) (Annamalai et al. 2023) that can in turn facilitate CIP degradation provided pH medium is favorable. In the final analysis, the photodegradation mechanism for CIP by 5CWT seems to occur via Z-scheme heterojunction with significant involvement of both the superoxide free radicals and holes.
Reusability and stability tests
The reusability tests and stability of 5CWT were evaluated by comparing UV–Vis DRS spectra of 120 ml containing 10 mg−1 CIP solution post 120 min of irradiation under visible light in the presence of 5CWT. The 5CWT was cleaned by centrifugation and oven dried at 80 °C for 24 h after each reusability test. The UV–Vis DRS analysis from Fig. 6a showed that 5CWT retained the photodegradation capacity for CIP but the efficiency reduced from 92% in cycle −1 to 68% in cycle 4. The reduced photocatalytic performance can be explained by the presence of C–C vibrations around 2300 cm−1 and C–H faint vibrations at 2931 cm−1 range on the FTIR spectra of loaded 5CWT in Fig. 6c (Janakiraman and Johnson 2015). The observable organic moiety vibrations are linked to the accumulated residue of degrading CIP byproducts adsorbed onto the surfaces of 5CWT with each of the four consecutive photodegradation cycles of CIP with a repeated 5CWT sample. These organic byproducts may obstruct a fraction of light from penetrating toward the 5CWT active sites and thereby inhabiting optimum photoactivity of the retained 5CWT photoactive functional groups. Despite the reduced light absorption intensity and mild appearance of C–C residue, the structural and functional changes of 5CWT were negligible. This means that the removal efficiencies in Fig. 6a and b can serve as evidence that 5CWT is a reusable photocatalyst while in Fig. 6c FTIR spectra serve as an attestation that 5CWT nanocomposite design is highly stable.
Proposed photodegradation pathways
Studies reported that LC–MS (Sarafraz et al. 2020) and LC–MS/MS (Zeng et al. 2019) techniques have an appreciable sensitivity to identify photodegraded ciprofloxacin byproducts. Figure 7 shows that CIP photodegradation pathways proceeded from four intermediate products: A = m/z 288, B = m/z 313, C = m/z 267, and D = m/z 314. The intermediate m/z 288 from pathway A is ascribed to a loss of CO2 (m/z 44) from the carboxylic group (decarboxylation) (Achad et al. 2018). This was followed by the mineralization of the piperazine ring, defluorination, and disintegration of the benzene ring to yield C2H5N, C2H5, F, and m/z 90 from m/z 197 byproducts. A previous study posted that a route that initiates piperazine ring cleavage before the defluorination step to yield intermediate m/z 245 proceeds through hydroxyl radicals (Zeng et al. 2019). The insignificant role of negatively charged hydroxyl radicals and the secondary role of negatively charged superoxide radicals in the current study imply that route A cannot be a major degradation pathway. Pathway B shows a probable degradation process proceeding in the order: of defluorination, then piperazine ring cleavage before decarboxylation. This is consistent with a previous study's argument that pathway B is probable at high pH (Vasconcelos et al. 2009). Pathway D shows that CIP m/z 332 is converted to m/z 314 by dehydration (loss of H2O = m/z 18) before it is converted into m/z 267 by losing epoxide, and to m/z 231 in pathway C by losing both C = O and fluorine group. Considering that; (i) pathway A—degradation initialized from m/z 288 but requires a significant OH participation, and (ii) pathway D initial sum loss of C = O then H2O can yield m/z 314 then m/z 288, it can be hypothesized that pathway D occurs before A. This concurs with the literature postulation that photolysis of CIP occurs within 2–4 min to give m/z 314 and m/z 288 as a major intermediate (Petrovi et al. 1067). This suggests that pathway D is a major pathway because initiating defluorination in pathway B requires more strength than dehydrating or decarboxylating CIP by removing a good leaving group—CO2H via pathway D. The LC–MS/MS results for the dissolved CIP by-products of photodegradation showed that 5CWT is capable of mineralizing CIP to molecular mass below 90 mol. g−1.
Comparative studies
Table 1 provides compares synthesized CuWO4-TiO2 (5CWT) to other TiO2-based nanocomposites applied for photocatalytic degradation of ciprofloxacin. The 5CWT displayed a superior performance for the photocatalytic degradation of CIP using an almost equivalent dosage to the reported composites. The 5CWT has an advantage because it performs under both visible light and abundantly available natural sunlight even at natural CIP solution pH in comparison with other catalysts that operate near neutral. The comparative studies complemented the findings that h+ is more involved in the CIP degradation mechanism than ·O2− radicals (Sarafraz et al. 2020; Ngo et al. 2023; Zeng et al. 2019). The observed large involvement of ·OH radical in other studies is associated with the near-neutral pHs used which provide zwitterionic speciation for CIP (Ngo et al. 2023; Zeng et al. 2019). With an exception for TiO2/CDs (Zeng et al. 2019), the Kapp for 5CWT is at least 40 folds higher than the other literature-reported catalysts. Even so, 5CWT still holds an advantage over TiO2/CDs since the current work rate constant value was determined using natural sunlight without adding external ozonation additives.
Conclusions
A novel sunlight-driven high charge separation heterogenous CuWO4-TiO2 (CWT) photocatalyst was sol–gel fabricated by reacting solvothermal-synthesized CuWO4-MOF nanopyramids and TiO2 nanoparticles precursors. The electron/hole charge separation was enhanced by rapid electron migration across heterojunction from TiO2 bands to CuWO4 conduction band. The enhanced charge separation allowed holes to dominantly initiate photocatalytic degradation of ciprofloxacin through four probable pathways. CWT loading of 5 mg CuWO4 displayed optimum 10 mg l−1 CIP photocatalytic degradation of 86% under visible light and ~ 100% under natural sunlight at pH = 9 for 2 h reaction time. Comparative studies showed that this recyclable novel CuWO4-TiO2 performed at a rate of 40 folds higher than the majority of recently manufactured TiO2-based nanocomposites for CIP degradation. This positions CWT as a promising heterojunction catalyst for use in the degradation of pharmaceutical pollutants under visible and natural sunlight conditions.
References
Achad M, Caumo S, de Castro Vasconcellos P, Bajano H, Gómez D, Smichowski P (2018) Chemical markers of biomass burning: determination of levoglucosan, and potassium in size-classified atmospheric aerosols collected in Buenos Aires, Argentina by different analytical techniques. Microchem J 139:181–187. https://doi.org/10.1016/j.microc.2018.02.016
Ambaye AD, Kefeni KK, Kebede TG, Ntsendwana B, Mishra SB, Nxumalo EN (2022) Cu-MOF/N-doped GO nanocomposites modified screen-printed carbon electrode towards detection of 4-nitrophenol. J Electroanal Chem 919:1–12. https://doi.org/10.1016/j.jelechem.2022.116542
Annamalai K, Natarajan S, Balakumar S (2023) Assisted assembling of Bi2WO6/rGO composites: a 3D/2D Hierarchical nanostructures for enhanced photocatalytic water remediation and photo-(electro)catalytic water splitting proficiency. Chemosphere 345:140488. https://doi.org/10.1016/j.chemosphere.2023.140488
Anucha CB, Altin I, Bacaksız E, Kucukomeroglu T, Belay MH, Stathopoulos VN (2021) Enhanced photocatalytic activity of CuWO4 doped TiO2 photocatalyst towards carbamazepine removal under UV irradiation. Separations 8:1–23. https://doi.org/10.3390/separations8030025
Bahramian H, Fattah-alhosseini A, Karbasi M, Nikoomanzari E, Giannakis S (2023) Synergy of Cu2+-Cu(OH)2-CuO with TiO2 coatings, fabricated via plasma electrolytic oxidation: insights into the multifaceted mechanism governing visible light-driven photodegradation of tetracycline. Chem Eng J 476:1–18. https://doi.org/10.1016/j.cej.2023.146588
Bai X, Fu Z, Ma X, Zhang Z, Fan J, Liu E, Li J (2022) Hydrophilic regulated photocatalytic converting phenol selectively over S-scheme CuWO4/TiO2. J Clean Prod 369:1–14. https://doi.org/10.1016/j.jclepro.2022.133099
Bao X, Wang X, Li X, Qin L, Han S, Kang SZ (2021) CuWO4-x nanoparticles incorporated brookite TiO2 porous nanospheres: preparation and dramatic photocatalytic activity for light driven H2 generation. Mater Res Bull 136:111171. https://doi.org/10.1016/j.materresbull.2020.111171
Barde A, Agbogo UV, Lawal AI (2022) Advances in the research and development of TiO2-based photocatalysts for hydrogen production via water splitting. Int J Multidiscip Res Growth Eval 3(4):1–8
Bharagav U, Reddy NR, Rao VN, Ravi P, Sathish M, Shankar MV, Kumari MM (2022) CuWO4 as a novel Z-scheme partner to construct TiO2 based stable and efficient heterojunction for photocatalytic hydrogen generation. Int J Hydrogen Energy 47:40391–40406. https://doi.org/10.1016/j.ijhydene.2022.07.155
Bibi S, Ahmad A, Anjum MAR, Haleem A, Siddiq M, Shah SS, Al Kahtani A (2021) Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J Environ Chem Eng 9:1–11. https://doi.org/10.1016/j.jece.2021.105580
De Luo B, Xiong XQ, Xu YM (2016) Improved photocatalytic activity for phenol degradation of rutile TiO2 on the addition of CuWO4 and possible mechanism. Wuli Huaxue Xuebao/acta Physico-Chimica Sinica 32:1758–1764. https://doi.org/10.3866/PKU.WHXB2016032805
Feng X, Chen Y, Wang M, Guo L (2017) Hydrothermal synthesis of pyramid-like In2S3 film for efficient photoelectrochemical hydrogen generation. Int J Hydrogen Energy 42:15085–15095. https://doi.org/10.1016/j.ijhydene.2017.04.283
Haghighi L, Haghnazari N, Karami C (2021) Tungsten disulfide quantum dots (WS2 QDs) as a fluorescence probe for detection of dopamine (DA). J Mater Sci: Mater Electron 32:28042–28050. https://doi.org/10.1007/s10854-021-07098-5
Honda K (1972) One and two-dimensional structure of alpha-helix and beta-sheet forms of poly(L-Alanine) shown by specific heat measurements at low temperatures (1.5-20 K). Nature 230:38–41
Hu X, Gao D, Li Y, Dong H, Zhou W, Yang L, Zhang Y (2019) Fabrication of novel CuWO4 nanoparticles (NPs) for photocatalytic degradation of methylene blue in aqueous solution. SN Appl Sci 1:119. https://doi.org/10.1007/s42452-018-0113-9
Jaegers NR, Lai J, He Y, Walter E, Dixon DA, Vasiliu M, Chen Y, Wang C, Hu MY, Mueller KT, Wachs IE, Wang Y, Hu JZ (2019) Heterogeneous catalysis mechanism by which tungsten oxide promotes the activity of supported V2O5/TiO2 catalysts for NOX abatement: structural effects revealed by 51 VMAS NMR spectroscopy. Angew Chem 131(36):12739–12746. https://doi.org/10.1002/ange.201904503
Janakiraman N, Johnson M (2015) Functional groups of tree ferns (Cyathea) using FTIR: chemotaxonomic implications. J Biophys 25(2):131–141
Kaur R, Kaur A, Kaur R, Singh S, Bhatti MS, Umar A, Baskoutas S, Kansal SK (2021) Cu-BTC metal organic framework (MOF) derived Cu-doped TiO2 nanoparticles and their use as visible light active photocatalyst for the decomposition of ofloxacin (OFX) antibiotic and antibacterial activity. Adv Powder Technol 32:1350–1361. https://doi.org/10.1016/j.apt.2021.02.037
Kovačević M, Živković S, Ognjanović M, Momčilović M, Relić D, Vasić Anićijević D (2023) In silico guided design of metal/semiconductor photocatalysts: a case of Cu-modified TiO2 for ciprofloxacin degradation. Materials 16:1–15. https://doi.org/10.3390/ma16165708
Liu Z, Song Q, Zhou M, Guo Z, Kang J, Yan H (2019) Synergistic enhancement of charge management and surface reaction kinetics by spatially separated cocatalysts and p-n heterojunctions in Pt/CuWO4/Co3O4 photoanode. Chem Eng J 374:554–563. https://doi.org/10.1016/j.cej.2019.05.191
Loka C, Gelija D, Vattikuti SVP, Lee KS (2023) Silver-boosted WO3/CuWO4 heterojunction thin films for enhanced photoelectrochemical water splitting efficiency. ACS Sustain Chem Eng 11:11978–11990. https://doi.org/10.1021/acssuschemeng.3c02150
MacWan DP, Dave PN, Chaturvedi S (2011) A review on nano-TiO2 sol-gel type syntheses and its applications. J Mater Sci 46:3669–3686. https://doi.org/10.1007/s10853-011-5378-y
Marschall R (2014) Semiconductor composites: strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv Funct Mater 24:2421–2440. https://doi.org/10.1002/adfm.201303214
Meenakshi GA, Sakthinathan S, Chiu TW (2023) Fabrication of carbon nanofiber incorporated with CuWO4 for sensitive electrochemical detection of 4-nitrotoluene in water samples. Sensors 23:1–15. https://doi.org/10.3390/s23125668
Mmelesi OK, Ammar-Merah S, Nkambule TTI, Kefeni KK, Kuvarega AT (2023) Synergistic role of N-doped carbon quantum dots on Zn-doped cobalt ferrite (N-CQDs/ZnCF) for the enhanced photodegradation of oxytetracycline under visible light. Mater Sci Eng B Solid State Mater Adv Technol 294:1–12. https://doi.org/10.1016/j.mseb.2023.116538
Moloto W, Mbule P, Nxumalo E, Ntsendwana B (2021) Stabilizing effects of zinc(II)-benzene-1,3,5-tricarboxylate metal organic frameworks on the performance of TiO2 photoanodes for use in dye-sensitized solar cells. J Photochem Photobiol A Chem 407:1–13. https://doi.org/10.1016/j.jphotochem.2020.113063
Nagakawa H, Nagata M (2021) Elucidating the factors affecting hydrogen production activity using a CdS/TiO2 type-II composite photocatalyst. ACS Omega 6:4395–4400. https://doi.org/10.1021/acsomega.0c05749
Nepfumbada C, Mamba BB, Bartlett BM, Fernández-Sánchez JF, Feleni U (2024) Lutetium copper@hexagonal boron nitride nanocomposite electrode system for sensing and signalling ciprofloxacin. ChemElectroChem 11(14):e202400168. https://doi.org/10.1002/celc.202400168
Ngo HS, Nguyen TL, Tran NT, Le HC (2023) Experimental study on kinetics and mechanism of ciprofloxacin degradation in aqueous phase using Ag-TiO2/rGO/halloysite photocatalyst. Catalysts 13:1–19. https://doi.org/10.3390/catal13020225
Pei L, Gu X, Zhang Y, Wang J, Tan H, Wang P, Zheng Z (2021) Construction of nano-TiO2 decorated titanosilicate core-shell structure: highly efficient oxygen activation for the degradation of Rhodamine B under visible light and excellent recycling performance. J Environ Chem Eng 9:1–10. https://doi.org/10.1016/j.jece.2021.105815
Petrovi M, Dolores Hernando M, Silvia Díaz-Cruz M, Barceló D (2005) Liquid chromatography-tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: a review. J Chromatogr A 1067:1–14. https://doi.org/10.1016/j.chroma.2004.10.110
Raizada P, Sharma S, Kumar A, Singh P, Khan AAP, Asiri AM (2020) Performance improvement strategies of CuWO4 photocatalyst for hydrogen generation and pollutant degradation. J Environ Chem Eng 8:1–25. https://doi.org/10.1016/j.jece.2020.104230
Ramezanalizadeh H, Manteghi F (2016) Synthesis of a novel MOF/CuWO4 heterostructure for efficient photocatalytic degradation and removal of water pollutants. J Clean Prod 172:2655–2666. https://doi.org/10.1016/j.jclepro.2017.11.145
Restrepo G, Valencia S, Marin JM (2010) Study of the bandgap of synthesized titanium dioxide nanoparticules using the sol-gel method and a hydrothermal treatment. Open Mater Sci J 4:9–14
Sarafraz M, Sadeghi M, Yazdanbakhsh A, Amini MM, Sadani M, Eslami A (2020) Enhanced photocatalytic degradation of ciprofloxacin by black Ti3+/N-TiO2 under visible LED light irradiation: kinetic, energy consumption, degradation pathway, and toxicity assessment. Process Saf Environ Prot 137:261–272. https://doi.org/10.1016/j.psep.2020.02.030
Sarwar A, Razzaq A, Zafar M, Idrees I, Rehman F, Kim WY (2023) Copper tungstate (CuWO4)/graphene quantum dots (GQDs) composite photocatalyst for enhanced degradation of phenol under visible light irradiation. Results Phys 45:1–11. https://doi.org/10.1016/j.rinp.2023.106253
Tan H, Gu X, Kong P, Lian Z, Li B, Zheng Z (2019) Cyano group modified carbon nitride with enhanced photoactivity for selective oxidation of benzylamine. Appl Catal B 242:67–75. https://doi.org/10.1016/j.apcatb.2018.09.084
Vasconcelos TG, Henriques DM, König A, Martins AF, Kümmerer K (2009) Photo-degradation of the antimicrobial ciprofloxacin at high pH: identification and biodegradability assessment of the primary by-products. Chemosphere 76:487–493. https://doi.org/10.1016/j.chemosphere.2009.03.022
Vinesh V, Preeyanghaa M, Kumar TRN, Ashokkumar M, Bianchi CL, Neppolian B (2022) Revealing the stability of CuWO4/g-C3N4 nanocomposite for photocatalytic tetracycline degradation from the aqueous environment and DFT analysis. Environ Res 207:1–12. https://doi.org/10.1016/j.envres.2021.112112
Wu JCS, Chen CH (2004) A visible-light response vanadium-doped titania nanocatalyst by sol-gel method. J Photochem Photobiol A Chem 163:509–515. https://doi.org/10.1016/j.jphotochem.2004.02.007
Xiong X, Chen H, Xu Y (2015) Improved photocatalytic activity of TiO2 on the addition of CuWO4. J Phys Chem C 119:5946–5953. https://doi.org/10.1021/jp510974f
Xue X, Weng Y, Yang S, Meng S, Zhang Z, Yi G, Zhang Y (2021) Effect of Cu-based metal organic framework (Cu-MOF) loaded with TiO2 on the photocatalytic degradation of rhodamine B dye. Environ Sci Pollut Res 28:15883–15889. https://doi.org/10.1007/s11356-020-11805-w/Published
Yuguru SP (2022) Unconventional reconciliation path for quantum mechanics and general relativity. IET Quantum Communication 3:99–111. https://doi.org/10.1049/qtc2.12034
Zeng Y, Chen D, Chen T, Cai M, Zhang Q, Xie Z, Li R, Xiao Z, Liu G, Lv W (2019) Study on heterogeneous photocatalytic ozonation degradation of ciprofloxacin by TiO2/carbon dots: Kinetic, mechanism and pathway investigation. Chemosphere 227:198–206. https://doi.org/10.1016/j.chemosphere.2019.04.039
Acknowledgements
This work was funded by National Research Funding (NRF) of South Africa, grant no. MND190409428558, and the Institute for Nanotechnology and Water Sustainability under the College of Science, Engineering, and Technology at the University of South Africa.
Funding
Open access funding provided by University of South Africa. National Research Foundation (RSA), MND190409428558, Kgaugelo S Mabape
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no known conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mabape, K.S., Mishra, S.B., Mishra, A.K. et al. Sunlight-driven charge separation for a heterojunction of nano-pyramidal CuWO4-MOF modified TiO2 nanoflakes for photocatalytic degradation of ciprofloxacin. Chem. Pap. 78, 8417–8432 (2024). https://doi.org/10.1007/s11696-024-03686-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03686-x